The Potential of Cocos nucifera Endocarp Carbon as an Adjunctive Root Canal Irrigant for Dentin Protection and Antibacterial Effects
Main Article Content
Abstract
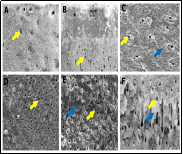
Enterococcus faecalis is a key pathogen in persistent endodontic infections, known for its resistance to conventional antimicrobial agents, including irrigants. Biocompatible alternatives that preserve dentin collagen are increasingly sought in root canal therapy. This study evaluated the antibacterial efficacy and dentin-preserving ability of coconut shell–derived activated carbon as an irrigant against E. faecalis. An ex vivo model using human premolars was infected with E. faecalis (ATCC 29212) for 21 days. Samples were treated with activated carbon (1:10, 1:20, 1:30), saline, or conventional irrigants (2% CHX, 17% EDTA, 2.5% NaOCl). Antibacterial activity was assessed via inhibition zones, CFU/mL, and OD600. Calcium ion release and hydroxyproline assays were used to evaluate demineralization and collagen degradation. SEM and FTIR (amide I and III) were used to assess the dentin structure and collagen integrity. Activated carbon 1:10 showed potent antibacterial activity (inhibition zone: 17.2 ± 0.4 mm) and CFU/mL reduction comparable to CHX. It had the lowest calcium release (3.9 ± 0.4 ppm) and hydroxyproline levels (4.2 ± 0.3 µg/mL), indicating minimal dentin damage. SEM and FTIR confirmed structural preservation. EDTA and NaOCl caused significant degradation of collagen. Coconut shell–derived activated carbon at a 1:10 concentration effectively inhibits E. faecalis while preserving dentin collagen, highlighting its novelty as a sustainable and biocompatible irrigant with potential clinical implications in root canal therapy.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Asmah N, Mattulada IK, Dodo AZA. Antibacterial assay of green tea (Camellia Sinensis L) against the growth of Enterococcus faecalis. J Syiah Kuala Dent Soc. 2023;8(2):211-6. DOI: https://doi.org/10.24815/jds.v8i2.36627
2. Darmawi I, Abidin T, Agusnar H, Gani BA. In Vitro Study of Irrigation solution of Chitosan Nanoparticles to Inhibit the Adhesion and Biofilm Formation of Enterococcus faecalis in the Root Canal. Res J Pharm Technol. 2022;15(6):2691-6. Doi:10.52711/0974-360X.2022.00450 DOI: https://doi.org/10.52711/0974-360X.2022.00450
3. Marques JA, Falacho RI, Santos JM, Ramos JC, Palma PJ. Effects of endodontic irrigation solutions on structural, chemical, and mechanical properties of coronal dentin: A scoping review. J Esthet Restor Dent. 2024;36(4):606-619. Doi: 10.1111/jerd.13135. DOI: https://doi.org/10.1111/jerd.13135
4. Boreak N, Al Mahde RZ, Otayn WA, Alamer AY, Alrajhi T, Jafri S, Sharwani A, Swaidi E, Abozoah S, Mowkly AAM. Exploring Plant-Based Compounds as Alternatives for Targeting Enterococcus faecalis in Endodontic Therapy: A Molecular Docking Approach. Int J Mol Sci. 2024;25(14):7727. Doi: 10.3390/ijms25147727.. DOI: https://doi.org/10.3390/ijms25147727
5. Pham TN, Nguyen TH, Le, VH, Nguyen TT, Nguyen TDK, Tran TN, Ho PAV, Co TT, Nguyen TTT, Vo TKA, Nguyen TH. Coconut shell-derived activated carbon and carbon nanotubes composite: a promising candidate for capacitive deionization electrode. Synth Met. 2020;265:116415. Doi.org/10.1016/j.synthmet.2020.116415 DOI: https://doi.org/10.1016/j.synthmet.2020.116415
6. Balasubramanian P. Valorization of biomass to activated carbon for wound dressing applications: Recent trends and future challenges. Bioresource Technol Rep. 2023;23:101562. Doi.org/10.1016/j.biteb.2023.101562 DOI: https://doi.org/10.1016/j.biteb.2023.101562
7. Rowińska I, Szyperska-Ślaska A, Zariczny P, Pasławski R, Kramkowski K, Kowalczyk P. The Influence of Diet on Oxidative Stress and Inflammation Induced by Bacterial Biofilms in the Human Oral Cavity. Materials (Basel). 2021;14(6):1444. Doi: 10.3390/ma14061444. DOI: https://doi.org/10.3390/ma14061444
8. Ghahramani Y, Fekri N, Mousavi SM, Hashemi SA, Lai CW. Green Carbon-Based Nanomaterials Against Dental Pathogens. Encyclopedia of Green Materials: Springer; 2023. p. 1-14. Doi.org/10.1007/978-981-16-4921-9_72-1 DOI: https://doi.org/10.1007/978-981-16-4921-9_72-1
9. Farghal N, Elkafrawy H. The effects of activated charcoal and calcium carbonate based toothpastes on surface properties of composite resin restorative materials. Egypt Dent J. 2020;66:2431-8. Doi.org/10.21608/edj.2020.35084.1170 DOI: https://doi.org/10.21608/edj.2020.35084.1170
10. Ioannidis K, Batty C, Turner C, Smith D, Mannocci F, Deb S. A laboratory study to assess the formation of effluent volatile compounds and disinfection by-products during chemomechanical preparation of infected root canals and application of activated carbon for their removal. Int Endod J. 2021;54(4):601-615. Doi: 10.1111/iej.13454. DOI: https://doi.org/10.1111/iej.13454
11. Baruwa AO, Martins JNR, Maravic T, Mazzitelli C, Mazzoni A, Ginjeira A. Effect of Endodontic Irrigating Solutions on Radicular Dentine Structure and Matrix Metalloproteinases-A Comprehensive Review. Dent J (Basel). 2022;10(12):219. Doi: 10.3390/dj10120219.
12. Widiyastuti W, Rois MF, Suari NMIP, Setyawan H. Activated carbon nanofibers derived from coconut shell charcoal for dye removal application. Adv Powder Technol. 2020;31(8):3267-73. Doi.org/10.1016/j.apt.2020.06.012. DOI: https://doi.org/10.1016/j.apt.2020.06.012
13. Kurmaena IE, Nurliza C, Gani BA. Effect of 17% ethylenediaminetetraacetic acid and silver citrate on sealer resin penetration in the apical third. Dent J. 2024;57(3):178-83. Doi:10.20473/j.djmkg.v57.i3.p178-183 DOI: https://doi.org/10.20473/j.djmkg.v57.i3.p178-183
14. Gani BA, Andayani R, Batubara FY, Ifwandi I, Syafriza D, Herlambang MMu, et al. Fungistatic effect of Gracilaria verrucosa on phospholipase enzymes and the cell surface hydrophobicity of Candida albicans. Dent J. 2025;58(1):66-73. Doi:10.20473/j.djmkg.v58.i1.p66-73 DOI: https://doi.org/10.20473/j.djmkg.v58.i1.p66-73
15. Sastika DY, Abidin T, Agusnar H, Gani BA. Application of calcium hydroxide with vehicles relate to the pH change, calcium ion diffusion, roughness, and frequency of chemical compound in root canal. Res J Pharm Technol. 2022;15(7):2976-82. Doi:0.52711/0974-360X.2022.00496 DOI: https://doi.org/10.52711/0974-360X.2022.00496
16. Gani A, Zakaria I, Gani BA, Diansari V. Toxicity Response of Chitosan Films on Wound Healing of Oral Mucosa Ephitelian Cells, In-Vivo and In-Silico Evaluation. Res J Pharma Technol. 2025;18(4):1795-804. Doi:10.52711/0974-360X.2025.00257 DOI: https://doi.org/10.52711/0974-360X.2025.00257
17. Ng HW, Zhang Y, Naffa R, Prabakar S. Monitoring the Degradation of Collagen Hydrogels by Collagenase Clostridium histolyticum. Gels. 2020;6(4):46. Doi: 10.3390/gels6040046. DOI: https://doi.org/10.3390/gels6040046
18. Syafriza D, Rifki A, Yulina V, Gani BA. The Assessment of Metabolic Changes and Stress Response of Streptococcus Mutans Growth in Saliva by Fourier Transform Infra-Red. J Int Dent Med Res. 2022;15(3):1086-94.
19. Mohammed H, Kumar A, Bekyarova E, Al-Hadeethi Y, Zhang X, Chen M, Ansari MS, Cochis A, Rimondini L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front Bioeng Biotechnol. 2020;8:465. Doi: 10.3389/fbioe.2020.00465. DOI: https://doi.org/10.3389/fbioe.2020.00465
20. Bhuiyan MSH, Miah MY, Paul SC, Aka TD, Saha O, Rahaman MM, Sharif MJI, Habiba O, Ashaduzzaman M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon. 2020;6(8):e04603. doi: 10.1016/j.heliyon.2020.e04603. DOI: https://doi.org/10.1016/j.heliyon.2020.e04603
21. Bapat RA, Parolia A, Chaubal T, Dharamadhikari S, Abdulla AM, Sakkir N, Arora S, Bapat P, Sindi AM, Kesharwani P. Recent update on potential cytotoxicity, biocompatibility and preventive measures of biomaterials used in dentistry. Biomater Sci. 2021;9(9):3244-3283. Doi: 10.1039/d1bm00233c.. DOI: https://doi.org/10.1039/D1BM00233C
22. Huang J, Luo J, Chen X, Feng S, Wan Y. How do chemical cleaning agents act on polyamide nanofiltration membrane and fouling layer? Ind Eng Chem Res. 2020;59(40):17653-70. Doi:10.1021/acs.iecr.0c03365 DOI: https://doi.org/10.1021/acs.iecr.0c03365
23. Nassar M, Nassar R, Maki H, Al-Yagoob A, Hachim M, Senok A, et al. Phytic acid: Properties and potential applications in dentistry. Front Mater. 2021;8:638909. Doi:10.3389/fmats.2021.638909 DOI: https://doi.org/10.3389/fmats.2021.638909
24. Giardino L, Savadori P, Generali L, Mohammadi Z, Del Fabbro M, De Vecchi E, Bidossi A. Antimicrobial effectiveness of etidronate powder (Dual Rinse® HEDP) and two EDTA preparations against Enterococcus faecalis: a preliminary laboratory study. Odontology. 2020;108(3):396-405. Doi: 10.1007/s10266-020-00499-8. DOI: https://doi.org/10.1007/s10266-020-00499-8
25. Liu H, Chen J, Qiao S, Zhang W. Carbon-Based Nanomaterials for Bone and Cartilage Regeneration: A Review. ACS Biomater Sci Eng. 2021;7(10):4718-4735. Doi: 10.1021/acsbiomaterials.1c00759. DOI: https://doi.org/10.1021/acsbiomaterials.1c00759
26. Omar N, Abdelraouf RM, Hamdy TM. Effect of different root canal irrigants on push-out bond strength of two novel root-end filling materials. BMC Oral Health. 2023 ;23(1):193. Doi: 10.1186/s12903-023-02858-7. DOI: https://doi.org/10.1186/s12903-023-02858-7
27. Barrera-Ortega CC, Rodil SE, Silva-Bermudez P, Delgado-Cardona A, Almaguer-Flores A, Prado-Prone G. Fluoride Casein Phosphopeptide and Tri-Calcium Phosphate Treatments for Enamel Remineralization: Effects on Surface Properties and Biofilm Resistance. Dent J (Basel). 2025;13(6):246. Doi: 10.3390/dj13060246. DOI: https://doi.org/10.3390/dj13060246
28. Baruwa AO, Martins JNR, Maravic T, Mazzitelli C, Mazzoni A, Ginjeira A. Effect of Endodontic Irrigating Solutions on Radicular Dentine Structure and Matrix Metalloproteinases-A Comprehensive Review. Dent J (Basel). 2022;10(12):219. Doi: 10.3390/dj10120219. DOI: https://doi.org/10.3390/dj10120219
29. Marrazzo P, O'Leary C. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering (Basel). 2020 ;7(3):104. doi: 10.3390/bioengineering7030104. DOI: https://doi.org/10.3390/bioengineering7030104
30. Rath PP, Yiu CKY, Matinlinna JP, Kishen A, Neelakantan P. The effects of sequential and continuous chelation on dentin. Dent Mater. 2020;36(12):1655-1665. Doi: 10.1016/j.dental.2020.10.010. DOI: https://doi.org/10.1016/j.dental.2020.10.010
31. Xiao J, Liu Y, Klein MI, Nikikova A, Ren Y. Nanotechnology and Delivery System for Bioactive Antibiofilm Dental Materials. Designing Bioactive Polymeric Materials For Restorative Dentistry: CRC Press; 2020. p. 165-97. Doi.org/10.1201/9780429113284-7 DOI: https://doi.org/10.1201/9780429113284-7
32. Kazemi-Yazdi H, Saeed-Nezhad M, Rezaei S. Effect of Chlorhexidine on durability of two self-etch adhesive systems. J Clin Exp Dent. 2020 ;12(7):e663-e669. Doi: 10.4317/jced.56873. DOI: https://doi.org/10.4317/jced.56873
33. Rónavári A, Igaz N, Adamecz DI, Szerencsés B, Molnar C, Kónya Z, Pfeiffer I, Kiricsi M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules. 2021 ;26(4):844. Doi: 10.3390/molecules26040844. DOI: https://doi.org/10.3390/molecules26040844
34. Al-Qudsy L, Hu YW, Xu H, Yang PF. Mineralized Collagen Fibrils: An Essential Component in Determining the Mechanical Behavior of Cortical Bone. ACS Biomater Sci Eng. 2023;9(5):2203-2219. Doi: 10.1021/acsbiomaterials.2c01377. DOI: https://doi.org/10.1021/acsbiomaterials.2c01377
35. Lutfi M, Hanafi, Bambang S, Prasetyo J, Malin Sutan S, Prajogo U. Characteristics of activated carbon from coconut shell (Cocos nucifera) through chemical activation process. IOP Conference Series: Earth and Environmental Science. 2021;733:012134. Doi.org/10.1088/1755-1315/733/1/012134. DOI: https://doi.org/10.1088/1755-1315/733/1/012134
36. Amin F, Fareed MA, Zafar MS, Khurshid Z, Palma PJ, Kumar N. Degradation and stabilization of resin-dentine interfaces in polymeric dental adhesives: an updated review. Coatings. 2022;12(8):1094. Doi.org/10.3390/coatings12081094 DOI: https://doi.org/10.3390/coatings12081094
37. Tartari T, Bachmann L, Zancan RF, Vivan RR, Duarte MAH, Bramante CM. Analysis of the effects of several decalcifying agents alone and in combination with sodium hypochlorite on the chemical composition of dentine. Int Endod J. 2018;51 Suppl 1:e42-e54. Doi: 10.1111/iej.12764. DOI: https://doi.org/10.1111/iej.12764
38. Cai C, Chen X, Li Y, Jiang Q. Advances in the Role of Sodium Hypochlorite Irrigant in Chemical Preparation of Root Canal Treatment. Biomed Res Int. 2023 ;2023:8858283. doi: 10.1155/2023/8858283. DOI: https://doi.org/10.1155/2023/8858283
39. Grawish ME, Grawish LM, Grawish HM, Grawish MM, Holiel AA, Sultan N, El-Negoly SA. Demineralized Dentin Matrix for Dental and Alveolar Bone Tissues Regeneration: An Innovative Scope Review. Tissue Eng Regen Med. 2022 Aug;19(4):687-701. Doi: 10.1007/s13770-022-00438-4. Epub 2022 Apr 16. Erratum in: Tissue Eng Regen Med. 2022 ;19(4):887-889. doi: 10.1007/s13770-022-00463-3. DOI: https://doi.org/10.1007/s13770-022-00463-3
40. Amirrah IN, Lokanathan Y, Zulkiflee I, Wee MFMR, Motta A, Fauzi MB. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines. 2022;10(9):2307. Doi: 10.3390/biomedicines10092307. DOI: https://doi.org/10.3390/biomedicines10092307
41. Mohamed AA, Fayyad DM, El-Telbany M, Mohamed DA. Antibacterial biofilm efficacy of calcium hydroxide loaded on Gum Arabic nanocarrier: an in-vitro study. BMC Oral Health. 2024;24(1):215. Doi: 10.1186/s12903-024-03941-3. DOI: https://doi.org/10.1186/s12903-024-03941-3
42. Yamin D, Uskoković V, Wakil AM, Goni MD, Shamsuddin SH, Mustafa FH, Alfouzan WA, Alissa M, Alshengeti A, Almaghrabi RH, Fares MAA, Garout M, Al Kaabi NA, Alshehri AA, Ali HM, Rabaan AA, Aldubisi FA, Yean CY, Yusof NY. Current and Future Technologies for the Detection of Antibiotic-Resistant Bacteria. Diagnostics (Basel). 2023 ;13(20):3246. Doi: 10.3390/diagnostics13203246. DOI: https://doi.org/10.3390/diagnostics13203246