Optimization of Polyphenol Extraction from Two Ziziphus Species via Central Composite Design-Response Surface Methodology: In Vitro Screening for Antioxidant, Antidiabetic, and Anti-Glycation Potentials
Main Article Content
Abstract
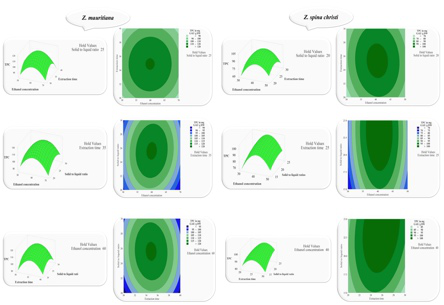
Polyphenols are widely available in medicinal plants and show promising pharmacological activity for many chronic diseases like diabetes mellitus. This study is aimed at optimizing the polyphenols from Ziziphus mauritiana and Ziziphus spina-christi using central composite design–response surface methodology (CCD–RSM) and evaluating their antioxidant, antidiabetic, and anti-glycation potentials. The key extraction parameters used for the optimal yield and biological evaluation were ethanol concentration, extraction time, solid-to-liquid ratio, and temperature. Total phenolic content (TPC) was maximized at intermediate ethanol concentrations (50–70% v/v), moderate extraction times (20–40 min), solid-to-liquid ratios of 20–25 g/mL, and temperatures between 40.0 and 60.0°C, and TPC obtained with optimal conditions was 120.59 mg GAE/g DW for Z. mauritiana and 104.77 mg GAE/g DW for Z. spina-christi. For the optimized extracts, Z. mauritiana showed significantly higher DPPH and ABTS (131.73 ± 7.23 mg and 271.62 ± 6.23 mg VCEAC/g DE) activities than Z. spina-christi (DPPH: 111.29 ± 4.34 mg; ABTS: 236.71 ± 8.23 mg VCEAC/g DE), while Z. spina-christi exhibited greater FRAP activity compared to Z. mauritiana (149.17 ± 6.23 mg and 123.53 ± 5.23 mg VCEAC/g DE, respectively). Z. spina-christi also had higher flavonoid and tannin content. In vitro assays revealed moderate α-amylase inhibition (IC₅₀ 112.30–176.20 µg/mL) and prominent anti-glycation effects (IC₅₀ 118–146 µg/mL), suggesting potential for managing oxidative stress and diabetes-related complications. This study underscores the phytotherapeutic potential of Ziziphus species and highlights CCD–RSM as an effective tool for optimizing bioactive compound extraction.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Sharma P, Hajam YA, Kumar R, Rai S. Complementary and alternative medicine for the treatment of diabetes and associated complications: A review on therapeutic role of polyphenols. Phytomed. Plus. 2022;2(1):100188. doi:10.1016/j.phyplu.2022.100188. DOI: https://doi.org/10.1016/j.phyplu.2021.100188
2. Anwar S, Khan S, Almatroudi A, Khan AA, Alsahli MA, Almatroodi SA, Rahmani AH. A review on mechanism of inhibition of advanced glycation end products formation by plant derived polyphenolic compounds. Mol Biol Rep. 2021;48(1):787-805. doi:10.1007/s11033-020-06084-0. DOI: https://doi.org/10.1007/s11033-020-06084-0
3. Nordin N, Abdullah AA, Ghani MF. Flavonoids exhibit potential antagonistic activity against platelet-activating factor (PAF) receptor. Trop J Nat Prod Res. 2022;6(10). doi: 10.26538/tjnpr/v6i10.11. DOI: https://doi.org/10.26538/tjnpr/v6i10.11
4. Bansal S, Burman A, Tripathi AK. Advanced glycation end products: Key mediator and therapeutic target of cardiovascular complications in diabetes. World J Diabetes. 2023;14(8):1146-1162. doi:10.4239/wjd.v14.i8.1146. DOI: https://doi.org/10.4239/wjd.v14.i8.1146
5. El Maaiden E, El Kharrassi Y, Qarah NAS, Essamadi AK, Moustaid K, Nasser B. Genus Ziziphus: A comprehensive review on ethnopharmacological, phytochemical and pharmacological properties. J Ethnopharmacol. 2020;259:112950. doi:10.1016/j.jep.2020.112950. DOI: https://doi.org/10.1016/j.jep.2020.112950
6. Toldra F. Advances in Food and Nutrition Research. Academic Press; 2025. 114 p.
7. Hussein AS. Ziziphus spina-christi: Analysis of bioactivities and chemical composition. In: Mariod, A. (eds). Wild Fruits: Composition, Nutritional Value and Products. Cham: Springer Int. Publ; 2019:175-197 p. doi: 10.1007/978-3-030-31885-7_15. DOI: https://doi.org/10.1007/978-3-030-31885-7_15
8. Roberts J, Dhileepan K, Florentine S. A review of the biology, distribution, and management challenges posed by the invasive weed Ziziphus mauritiana L., with special reference to its invasion in Australia. Weed Res. 2024;64(1):8-18. doi: 10.1111/wre.12610. DOI: https://doi.org/10.1111/wre.12610
9. Al-Fatimi M. Wild edible plants traditionally collected and used in southern Yemen. J Ethnobiol Ethnomed. 2021;17(1):49. doi:10.1186/s13002-021-00475-8. DOI: https://doi.org/10.1186/s13002-021-00475-8
10. Butt SZ, Hussain S, Munawar KS, Tajammal A, Muazzam MA. Phytochemistry of Ziziphus mauritiana; its nutritional and pharmaceutical potential. Sci Inq Rev. 2021;5(2):1-15. doi: 10.32350/sir.52.01. DOI: https://doi.org/10.32350/sir.52.01
11. Abdulrahman MD, Zakariya AM, Hama HA, Hamad SW, Al-Rawi SS, Bradosty SW, Ibrahim AH. Ethnopharmacology, biological evaluation, and chemical composition of Ziziphus spina-christi (L.) Desf.: A review. Adv Pharmacol Pharm Sci. 2022;2022:4495688. doi:10.1155/2022/4495688. DOI: https://doi.org/10.1155/2022/4495688
12. Yahia Y, Benabderrahim MA, Tlili N, Bagues M, Nagaz K. Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two Ziziphus Mill. species. PLoS One. 2020;15(5):e0232599. doi:10.1371/journal.pone.0232599. DOI: https://doi.org/10.1371/journal.pone.0232599
13. Lestari DY, Mastutik G, Mukono IS. Ziziphus mauritiana in triple-negative breast cancer: Integrating network pharmacology and in vitro evaluation. Trop J Nat Prod Res. 2025;9(1). doi: 10.26538/tjnpr/v9i1.28. DOI: https://doi.org/10.26538/tjnpr/v9i1.28
14. Palos-Hernandez A, Gonzalez-Paramas AM, Santos-Buelga C. Latest advances in green extraction of polyphenols from plants, foods and food by-products. Molecules. 2024;30(1):55. doi:10.3390/molecules30010055. DOI: https://doi.org/10.3390/molecules30010055
15. Makkiyah FA, Pradana DL, Putra RP, Nurcholis W. Optimization microwave-assisted extraction of Moringa oleifera leaves using response surface methodology focused on extracting phenolic and flavonoid with antioxidant activity. Trop J Nat Prod Res. 2024;8(6). doi: 10.26538/tjnpr/v8i6.21. DOI: https://doi.org/10.26538/tjnpr/v8i6.21
16. Didion YP, Tjalsma TG, Su Z, Malankowska M, Pinelo M. What is next? The greener future of solid-liquid extraction of biobased compounds: Novel techniques and solvents overpower traditional ones. Sep Purif Technol. 2023;320:124147. doi: 10.1016/j.seppur.2023.124147. DOI: https://doi.org/10.1016/j.seppur.2023.124147
17. Sri KA, Kamiliyah HN, Runadi D. Enhanced extraction of antishigellosis compounds from Ficus elastica leaves: A response surface methodology approach. Trop J Nat Prod Res. 2025;9(2). doi: 10.26538/tjnpr/v9i2.32. DOI: https://doi.org/10.26538/tjnpr/v9i2.32
18. Cannavacciuolo C, Pagliari S, Celano R, Campone L, Rastrelli L. Critical analysis of green extraction techniques used for botanicals: Trends, priorities, and optimization strategies—A review. TrAC Trends Anal Chem. 2024:117627. doi: 10.1016/j.trac.2024.117627. DOI: https://doi.org/10.1016/j.trac.2024.117627
19. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16(3):144-158. doi: 10.5344/ajev.1965.16.3.144. DOI: https://doi.org/10.5344/ajev.1965.16.3.144
20. Annegowda HV, Bhat R, Min-Tze L, Karim AA, Mansor SM. Influence of sonication treatments and extraction solvents on the phenolics and antioxidants in star fruits. J Food Sci Technol. 2012;49(4):510-514. doi:10.1007/s13197-011-0435-8. DOI: https://doi.org/10.1007/s13197-011-0435-8
21. Brand-Williams W, Cuvelier M-E, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25-30. doi: 10.1016/S0023-6438(95)80008-5. DOI: https://doi.org/10.1016/S0023-6438(95)80008-5
22. Annegowda H, Bhat R, Yeong KJ, Liong M-T, Karim A, Mansor S. Influence of drying treatments on polyphenolic contents and antioxidant properties of raw and ripe papaya (Carica papaya L.). Int J Food Prop. 2014;17(2):283-292. doi: 10.1080/10942912.2011.631248. DOI: https://doi.org/10.1080/10942912.2011.631248
23. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9-10):1231-1237. doi:10.1016/S0891-5849(98)00315-3. DOI: https://doi.org/10.1016/S0891-5849(98)00315-3
24. Broadhurst RB, Jones WT. Analysis of condensed tannins using acidified vanillin. J Sci Food Agric. 1978;29(9):788-794. doi: 10.1002/jsfa.2740290908. DOI: https://doi.org/10.1002/jsfa.2740290908
25. Bhat R, Sridhar KR, Tomita-Yokotani K. Effect of ionizing radiation on antinutritional features of velvet bean seeds (Mucuna pruriens). Food Chem. 2007;103(3):860-866. doi: 10.1016/j.foodchem.2006.09.037. DOI: https://doi.org/10.1016/j.foodchem.2006.09.037
26. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239(1):70-76. doi: 10.1006/abio.1996.0292. DOI: https://doi.org/10.1006/abio.1996.0292
27. Chang C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3). doi: 10.38212/2224-6614.2748. DOI: https://doi.org/10.38212/2224-6614.2748
28. Lankatillake C, Luo S, Flavel M, Lenon GB, Gill H, Huynh T, Dias DA. Screening natural product extracts for potential enzyme inhibitors: Protocols, and the standardisation of the usage of blanks in alpha-amylase, alpha-glucosidase and lipase assays. Plant Methods. 2021;17(1):3. doi:10.1186/s13007-020-00702-5. DOI: https://doi.org/10.1186/s13007-020-00702-5
29. Dos Santos FAR, Xavier JA, da Silva FC, Merlin JPJ, Goulart MOF, Rupasinghe HPV. Antidiabetic, antiglycation, and antioxidant activities of ethanolic seed extract of Passiflora edulis and piceatannol in vitro. Molecules. 2022;27(13):4064. doi:10.3390/molecules27134064 DOI: https://doi.org/10.3390/molecules27134064
30. Klongdee S, Klinkesorn U. Optimization of accelerated aqueous ethanol extraction to obtain a polyphenol-rich crude extract from rambutan (Nephelium lappaceum L.) peel as natural antioxidant. Sci Rep. 2022;12(1):21153. doi: 10.1038/s41598-022-25818-7. DOI: https://doi.org/10.1038/s41598-022-25818-7
31. Aghoutane B, Talbi H, Naama A, El Monfalouti H, Kartah BE. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Euphorbia resinifiera O. Berg. Trop J Nat Prod Res. 2023;7(3). doi: 10.26538/tjnpr/v7i3.10. DOI: https://doi.org/10.26538/tjnpr/v7i3.10
32. Ahmad I, Narsa AC, Ramadhani MR, Zamruddin NM, Iswahyudi I, Hajrah H, Indriyanti N, Arifuddin M, Siska S, Supandi S, Ambarwati NSS. Optimization of microwave-assisted extraction on polyphenol metabolite from Eleutherine bulbosa (Mill.) Urb. bulbs using response surface methodology. J Adv Pharm Technol Res. 2023;14(2):113-118. doi:10.4103/japtr.japtr_613_22. DOI: https://doi.org/10.4103/japtr.japtr_613_22
33. Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem. 2017;34:540-560. doi:10.1016/j.ultsonch.2016.06.035. DOI: https://doi.org/10.1016/j.ultsonch.2016.06.035
34. Ponticelli M, Carlucci V, Mecca M, Todaro L, Milella L, Russo D. Extraction optimization of Quercus cerris L. wood chips: A comparative study between full factorial design (FFD) and artificial neural network (ANN). Antioxidants. 2024;13(9):1115. doi: 10.3390/antiox13091115. DOI: https://doi.org/10.3390/antiox13091115
35. Savikin K, Zivkovic J, Jankovic T, Cujic-Nikolic N, Zdunic G, Menkovic N, Drinic Z. Optimization of ultrasound-assisted extraction of phenolics from Sideritis raeseri using response surface methodology. Molecules. 2021;26(13):3949. doi:10.3390/molecules26133949. DOI: https://doi.org/10.3390/molecules26133949
36. Antony A, Farid M. Effect of temperatures on polyphenols during extraction. Appl Sci. 2022;12(4):2107. doi: 10.3390/app12042107. DOI: https://doi.org/10.3390/app12042107
37. Khanam A, Ijaz Hussain A, Mohammed EH, Nahar L, Rathore HA. Phenolic profile of seedless Ziziphus mauritiana fruits and leaves extracts with in vivo antioxidant and anti-inflammatory activities: Influence on pro-inflammatory mediators. Chem Biodivers. 2025;22(3):e202401728. doi:10.1002/cbdv.202401728. DOI: https://doi.org/10.1002/cbdv.202401728
38. Ambrin A, Adil M, Filimban FZ, Naseer M. Chemical profiling and biological activities of Ziziphus mauritiana var. spontanea (Edgew.) RR Stewart ex Qaiser & Nazim. and Oenothera biennis L. J Food Qual. 2024;2024:7318407. doi: 10.1155/2024/7318407. DOI: https://doi.org/10.1155/2024/7318407
39. Khaleel SM, Jaran AS, Haddadin MS. Evaluation of total phenolic content and antioxidant activity of three leaf extracts of Ziziphus spina-christi (Sedr) grown in Jordan. Br J Med Med Res. 2016;14(6):1-8. doi: 10.9734/BJMMR/2016/24935. DOI: https://doi.org/10.9734/BJMMR/2016/24935
40. Jabba HL, Dimeji IY, Babatunde AA, Baba ZM, Ayodeji AS, Adeoye SW. Evaluation of anxiolytic and behavioral activity of ethyl acetate leaf extract of Ziziphus spina-christi leaves in Swiss albino mice. Trop J Nat Prod Res. 2025;9(4). doi: 10.26538/tjnpr/v9i4.42. DOI: https://doi.org/10.26538/tjnpr/v9i4.42
41. Geidam YA, Daja A, Ngulde SI, Usman H, Gidado A. Anti-nociceptive activity of combined methanol extract of edible fruit pulps of some medicinal plants. Trop J Nat Prod Res. 2019;4(4):136-140. doi:10.26538/tjnpr/v4i4.4. DOI: https://doi.org/10.26538/tjnpr/v4i4.4
42. Youl ENH, Ouedraogo CAP, Gambo M, Ouedraogo M, Kiendrebeogo M, Traore A, Guissou IP. Antioxidant activity of crude ethanolic extract and fractions of Ziziphus mauritiana Lam. (Rhamnaceae) leaves from Burkina Faso. J Basic Clin Physiol Pharmacol. 2019;30(3):20170176. doi:10.1515/jbcpp-2017-0176. DOI: https://doi.org/10.1515/jbcpp-2017-0176
43. Boakye-Gyasi E, Henneh IT, Abotsi WKM, Ameyaw EO, Woode E. Hydro-ethanolic leaf extract of Ziziphus abyssinica Hochst ex A. Rich (Rhamnaceae) exhibits anti-nociceptive effects in murine models. BMC Complement Altern Med. 2017;17(1):231. doi:10.1186/s12906-017-1750-z. DOI: https://doi.org/10.1186/s12906-017-1750-z
44. Jain P, Haque A, Islam T, Alam MA, Reza HM. Comparative evaluation of Ziziphus mauritiana leaf extracts for phenolic content, antioxidant and antibacterial activities. J Herbs Spices Med Plants. 2019;25(3):236-258. doi: 10.1080/10496475.2019.1600627. DOI: https://doi.org/10.1080/10496475.2019.1600627
45. El-Shahir AA, El-Wakil DA, Abdel Latef AAH, Youssef NH. Bioactive compounds and antifungal activity of leaves and fruits methanolic extracts of Ziziphus spina-christi L. Plants (Basel). 2022;11(6):746. doi:10.3390/plants11060746. DOI: https://doi.org/10.3390/plants11060746
46. Iheanacho CM, Akubuiro PC, Oseghale IO, Imieje VO, Erharuyi O, Ogbeide KO, Jideonwo AN, Falodun A. Evaluation of the antioxidant activity of the stem bark extracts of Anacardium occidentale (Linn) Anacardiaceae. Trop J Phytochem Pharm Sci. 2023;2(2):65-69. doi: 10.26538/tjpps/v2i2.4. DOI: https://doi.org/10.26538/tjpps/v2i2.4
47. Egharevba E, Chukwuemeke-Nwani P, Eboh U, Okoye E, Bolanle IO, Oseghale IO, Imieje VO, Erharuyi O, Falodun A. Evaluation of the antioxidant and hypoglycaemic potentials of the leaf extracts of Stachytarphyta jamaicensis (Verbenaceae). 2019. doi: 10.26538/tjnpr/v3i5.4. DOI: https://doi.org/10.26538/tjnpr/v3i5.4
48. Okolie NP, Falodun A, Davids O. Evaluation of the antioxidant activity of root extract of pepper fruit (Dennetia tripetala), and its potential for the inhibition of lipid peroxidation. Afr J Tradit Complement Altern Med. 2014;11(3):221-227. doi: 10.4314/ajtcam.v11i3.31. DOI: https://doi.org/10.4314/ajtcam.v11i3.31
49. Imran S, Bibi Y, Munawar T, Yousaf AM, Hasnain M. A panoramic review on ethnomedicinal, therapeutic, phytochemical, and advance attributes of the genus Ziziphus Mill., native to Pakistan. Ethnobot Res Appl. 2023;25:1-32. doi: 0.32859/era.25.67.1-31. DOI: https://doi.org/10.32859/era.25.67.1-31
50. Adilah HN, Saleh MI, Az-Zahra NDA, Cho E, Sinaga E. Total phenolic and total flavonoid content, antioxidant activity, and nutritional profile of Ziziphus mauritiana fruit juice. Int J Biol Phys Chem Stud. 2023;5(1):1-8. doi: 10.32996/ijbpcs. DOI: https://doi.org/10.32996/ijbpcs.2023.5.1.1
51. Al-Ghamdi AAM, El-Zohri M, Shahat AA. Hepatoprotective, nephroprotective, anti-amylase, and antiglucosidase effects of Ziziphus spina-christi (L.) against carbon tetrachloride-induced toxicity in rats. Trop J Pharm Res. 2019;18(4):781-790. doi: 10.4314/tjpr.v18i4.15. DOI: https://doi.org/10.4314/tjpr.v18i4.15
52. Junedi S, Nurwijayanto A, Simamora DD, Palimbongan AM, Arsiningtyas IS. Potential extracts of Melastomataceae species from Mount Merapi National Park as sun protection material with antioxidation and antiglycation activities. Trop J Nat Prod Res. 2023;7(1). doi: 10.26538/tjnpr/v7i1.14. DOI: https://doi.org/10.26538/tjnpr/v7i1.14
53. Khan I, Zahoor M, Zeb A, Sahibzada MUK, Bari WU, Naz S. Isolation, characterization, pharmacological evaluation and in silico modeling of bioactive secondary metabolites from Ziziphus oxyphylla, a member of Rhamnaceae family. Trop J Pharm Res. 2020;19(2):351-359. doi: 10.4314/tjpr.v19i2.18. DOI: https://doi.org/10.4314/tjpr.v19i2.18