Optimization of Microwave - Assisted Extraction Conditions for Total Saponin Content and Antioxidant Activity of Launaea sarmentosa Leaves
Main Article Content
Abstract
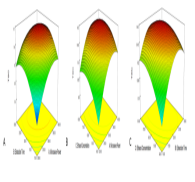
Launaea sarmentosa is an herbaceous plant rich in saponin, especially in the leaves. The plant has been shown to have antioxidant activity. The present study aimed to optimize the extraction conditions for maximizing both total saponin content (TSC) and antioxidant activity of the plant leaves. To achieve this, Response Surface Methodology (RSM) was applied using a Box-Behnken Design (BBD), which involved three independent variables: microwave power, extraction time, and ethanol concentration. These parameters were systematically optimized to maximize the extraction yield of the desired bioactive compounds. TSC was determined spectrophotometrically using diosgenin as the reference standard. Antioxidant activity was assessed through the DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging method. The optimal conditions determined through the model were microwave power of 330 W, extraction time of 22 minutes, and ethanol concentration of 64%. Under these conditions, the experimental values of TSC and antioxidant activity were found to be 9.56 ± 0.24 mg DE/g dry weight and 87.62 ± 0.46%, respectively. The findings indicate that microwave-assisted extraction, when optimized using RSM, is an effective approach for enhancing the recovery of bioactive components from L. sarmentosa. This study provides a scientific basis for the efficient use of this plant as a natural source of antioxidants. In addition, the results gave valuable insights into the development of plant-based functional ingredients for pharmaceutical and nutraceutical applications. By optimizing the extraction conditions, this work demonstrated the potential of Launaea sarmentosa as a promising candidate for use in health-promoting products, paving the way for its broader industrial application.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Moghal MMR, Millat MS, Hussain MS, Islam MR. Thrombolytic and Membrane Stabilizing Activities of Launaea sarmentosa. Int J Pharmacogn. 2016; 3:354-358. doi: 10.13040/IJPSR.0975-8232.IJP.3(8).354-58. DOI: https://doi.org/10.13040/IJPSR.0975-8232.IJP.3(8).354-58
2.Millat MS, Islam S, Hussain SM, Moghal MR, Islam T. Anti-Bacterial Profiling of Launaea sarmentosa (Willd.) and Bruguiera cylindrical (L.): Two Distinct Ethno Medicinal Plants of Bangladesh. Eur J Exp Biol. 2017; 7:1-6. doi: 10.21767/2248-9215.100006. DOI: https://doi.org/10.21767/2248-9215.100006
3.Abdelkrim C, Mebarka B, Nasser B, Houria D. Phytochemical and Biological Studies on Launaea Cass. Genus (Asteraceae) From Algerian Sahara. Curr Top Phytochem. 2012; 11:67-80.
4.Yusriyya S, Harischa CR, Vinay JS, Rabinarayan A. Pharmacognostical Evaluation of Launaea sarmentosa (Willd.) Schultz-Bip. ex Kuntze Root. An Int Q J Res Ayurveda. 2013; 34:90-94. doi: 10.4103/0974-8520.115439. DOI: https://doi.org/10.4103/0974-8520.115439
5.Hao JY, Han W, Huang SD, Xue BY, Deng X. Microwave-Assisted Extraction of Artemisinin from Artemisia annua L. Sep Purif Technol. 2002; 28:191–196. doi: 10.1016/S1383-5866(02)00043-6. DOI: https://doi.org/10.1016/S1383-5866(02)00043-6
6.Kratchanova M, Panchev I, Pavlova E. The Effect of Microwave Heating of Fresh Orange Peels on The Fruit Tissue and Quality of Extracted Pectin. Carbohydr Polym. 2004; 56:181–185. doi: 10.1016/j.carbpol.2004.01.009. DOI: https://doi.org/10.1016/j.carbpol.2004.01.009
7.Bas D and Boyaci IH. Modeling and Optimization I: Usability of Response Surface Methodology. J Food Eng. 2007; 788:836–845. doi: 10.1016/j.jfoodeng.2005.11.024. DOI: https://doi.org/10.1016/j.jfoodeng.2005.11.024
8.Them LT, Tuan NT, Trinh PTN, Tho LTC, Vi LNT, Hung QT, Dung NPT, Thuy NV, Dung LT. Saponin, Polyphenol, Flavonoid Content and α-Glucosidase Inhibitory Activity, Antioxidant Potential of Launaea sarmentosa Leaves Grown in Ben Tre Province, Vietnam. IOP Conf Ser Mater Sci Eng. 2019; 542(1):012036. doi: 10.1088/1757-899X/542/1/012036. DOI: https://doi.org/10.1088/1757-899X/542/1/012036
9.Hu T, Guo YY, Zhou QF, Zhong XK, Zhu L, Piao JH, Chen J, Jiang JG. Optimization of Ultrasonic-Assisted Extraction of Total Saponins From Eclipta prostrasta L. Using Response Surface Methodology. J Food Sci. 2012; 77:975-982. doi: 10.1111/j.1750-3841.2012.02869.x. DOI: https://doi.org/10.1111/j.1750-3841.2012.02869.x
10.Moyo M, Amoo SO, Ncube B, Ndhlala AR, Finnie JF, Van Staden J. Phytochemical and Antioxidant Properties of Unconventional Leafy Vegetables Consumed in Southern Africa. S Afr J Bot. 2013; 84:65-71. doi: 10.1016/j.sajb.2012.09.010. DOI: https://doi.org/10.1016/j.sajb.2012.09.010
11.Rajha HN, El Darra N, Hobaika Z, Boussetta N, Vorobiev E, Maroun RG, Louka N. Extraction of Total Phenolic Compound, Favonoids, Anthocyanins, and Tannins from Grape Byproducts by Response Surface Methodology. Influence of Solid-Liquid Ratio, Particle Size, Time, Temperature and Solvent Mixtures on the Optimization Process. Food Nutr Sci. 2014; 5:397–409. doi: 10.4236/fns.2014.54048. DOI: https://doi.org/10.4236/fns.2014.54048
12.Akbari S, Abdurahman NH, Yunus RM. Optimization of Saponins, Phenolics, and Antioxidants Extracted from Fenugreek Seeds Using Microwave-Assisted Extraction and Response Surface Methodology as An Optimizing Tool. C R Chim. 2019; 22:714-727. doi: 10.1016/j.crci.2019.07.007. DOI: https://doi.org/10.1016/j.crci.2019.07.007
13.Xu HJ, Shi XW, Ji X, Du YF, Zhu H, Zhang LT. A Rapid Method for Simultaneous Determination of Triterpenoid Saponins In Pulsatilla turczaninovii Using Microwave-Assisted Extraction and High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Food Chem. 2012; 135:251-258. doi: 10.1016/j.foodchem.2012.04.081. DOI: https://doi.org/10.1016/j.foodchem.2012.04.081
14.Amid BT and Mirhosseini H. Effect of Different Purification Techniques on The Characteristics of Heteropolysaccharide-Protein Biopolymer from Durian (Durio zibethinus) Seed. Molecules. 2012; 17:10875-10892. doi: 10.3390/molecules170910875. DOI: https://doi.org/10.3390/molecules170910875
15.Thanh NT. Optimization of Microwave-Assisted Extraction of Antioxidant Compounds from Roots of Polygonum multiflorum Thunb. At Vietnam Using Response Surface Methodology. Malays J Chem. 2023; 25:21-29. DOI: https://doi.org/10.55373/mjchem.v25i4.21
16.Chi TP, Trang BT, Trinh NTN, Tho LTC, Thanh NT, Thang TD, Hang HTL, Quang LD, Tuan NN. Optimization of Ultrasonic-Assisted Extraction of Antioxidant Compounds in Black Shallots (Allium ascalonicum) From Vietnam Using Response Surface Methodology. Malays J Chem. 2022; 24:26-35. DOI: https://doi.org/10.55373/mjchem.v24i1.1264