Phaleria Macrocarpa Extract Reduced Endometriosis Lesions by Regulating Inflammatory Protein and Inhibiting Ki67 and Vegf-A Protein
Main Article Content
Abstract
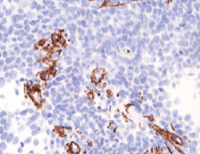
Endometriosis is a complex and multifactorial disease causing severe pelvic pain, infertility, and reduced quality of life in women. Therefore, this study aimed to evaluate the preventive potential of Phaleria macrocarpa (PM) mesocarp ethanol extract on endometriosis. The animals used for the experiment were divided into eight groups, each comprising four mice. The first group served as control comprising healthy mice without PM treatment and adenomyosis (ADM) implantation. The second group was the control negative consisting of mice with ADM implantation and without PM treatment. The remaining six groups consisted of ADM mice treated with several doses of PM including 3.75, 7.5, 11.25, 15, 18.75, and 22.5 mg/kg/day orally for 14 days. On day 1 PM treatment, endometriosis was induced by implanting ADM tissue intraperitoneally in mice given an immunosuppressant, cyclosporin. Furthermore, mice were injected with estradiol intramuscularly (54 IU/mice) on the first and fifth day after ADM implantation. At day 15, the samples were sacrificed, endometriosis tissue was isolated to measure the implant area, and the ascites of peritoneal were collected to evaluate the level of Cyclooxygenase-2 (COX-2). The evaluation was performed using Enzyme-Linked Immunosorbent Assay (ELISA), and Nuclear Factor kappa-light-chain-enhancer of activated B cells p65 subunit (NF-κB p65), Vascular Endothelial Growth Factor A (VEGF-A), and Kiel-67 (Ki67) of peritoneal tissue expression level by immunohistochemistry. The results showed that PM could prevent inflammation and the development of endometriosis by reducing the level of COX-2 in a dose-dependent method. Additionally, PM extract inhibited the expression level of NF-κB p65, VEGF-A, and Ki67, reducing the ADM implant area.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Allaire C, Bedaiwy MA, Yong PJ. Diagnosis and management of endometriosis. Can. Med. Assoc. J. 2023;195(7):363–371. Doi: 10.1503/cmaj.220637 DOI: https://doi.org/10.1503/cmaj.220637
2.Ochoa Bernal MA, Fazleabas AT. The Known, the Unknown and the Future of the Pathophysiology of Endometriosis. Int J Mol Sci. 2024;25(11):1-34. Doi: 10.3390/ijms25115815. DOI: https://doi.org/10.3390/ijms25115815
3.Chauhan S, More A, Chauhan V, Kathane A. Endometriosis: A Review of Clinical Diagnosis, Treatment, and Pathogenesis. Cureus. 2022. Doi: 10.7759/cureus.28864. DOI: https://doi.org/10.7759/cureus.28864
4.Horne AW, Missmer SA. Pathophysiology, diagnosis, and management of endometriosis. BMJ. 2022;379:e070750. Doi: 10.1136/bmj-2022-070750. DOI: https://doi.org/10.1136/bmj-2022-070750
5.Cano-Herrera G, Nehmad SS, Gascón JRC, Gascón JRC, Vionet AM, van Tienhoven XA, Martínez MFO, Alvarez MM, Rivero MXV, Torres MFL, Valverde MJB, Torres IN, Olascoaga AC, Gonzalez MFB, Nehme JAS, Rodríguez IV, Pérez RM, Salazar FE, Bronzon AGS, Rosas EGR, Ocampo DC, Carranco RC. Endometriosis: A Comprehensive Analysis of the Pathophysiology, Treatment, and Nutritional Aspects, and Its Repercussions on the Quality of Life of Patients. Biomedicines. 2024;12(7):1476. Doi: 10.3390/biomedicines12071476. DOI: https://doi.org/10.3390/biomedicines12071476
6.Zhang H, Sheng S, Pan Z, Zhao L, Yang C, Li C, Wang F. Immune and endocrine regulation in endometriosis: what we know. J Endometriosis Uterine Disord. 2023;4:100049. Doi: 10.1016/j.jeud.2023.100049. DOI: https://doi.org/10.1016/j.jeud.2023.100049
7.Abramiuk M, Grywalska E, Małkowska P, Sierawska O, Hrynkiewicz R, Niedźwiedzka-Rystwej P. The Role of the Immune System in the Development of Endometriosis. Cells. 2022;11(13):2028. Doi: 10.3390/cells11132028. DOI: https://doi.org/10.3390/cells11132028
8.Donnez J, Cacciottola L. Endometriosis: An Inflammatory Disease That Requires New Therapeutic Options. Int J Mol Sci. 2022;23(3):1518. Doi: 10.3390/ijms23031518. DOI: https://doi.org/10.3390/ijms23031518
9.Zdrojkowski Ł, Jasiński T, Ferreira-Dias G, Pawliński B, Domino M. The Role of NF-κB in Endometrial Diseases in Humans and Animals: A Review. Int J Mol Sci. 2023;24(3):2901. Doi: 10.3390/ijms24032901. DOI: https://doi.org/10.3390/ijms24032901
10.Liu Y, Wang J, Zhang X. An Update on the Multifaceted Role of NF-kappaB in Endometriosis. Int J Biol Sci. 2022;18(11):4400–4413. Doi: 10.7150/ijbs.72707. DOI: https://doi.org/10.7150/ijbs.72707
11.Barbara G, Buggio L, Facchin F, Vercellini P. Medical Treatment for Endometriosis: Tolerability, Quality of Life and Adherence. Front Glob Women’s Health. 2021;2:729601. Doi: 10.3389/fgwh.2021.729601. DOI: https://doi.org/10.3389/fgwh.2021.729601
12.Nezhat C, Vang N, Tanaka PP, Nezhat C. Optimal Management of Endometriosis and Pain. Obstet Gynecol. 2019;134(4):834–839. Doi: 10.1097/AOG.0000000000003461. DOI: https://doi.org/10.1097/AOG.0000000000003461
13.Yin WW, Huang CC, Chen YR, Yu DQ, Jin M, Feng C. The effect of medication on serum anti-müllerian hormone (AMH) levels in women of reproductive age: a meta-analysis. BMC Endocr Disord. 2022;22(1):158. Doi: 10.1186/s12902-022-01065-9. DOI: https://doi.org/10.1186/s12902-022-01065-9
14.Maharani M. Phaleria macrocarpa for Endometriosis Treatment: A Review. J Med Pharm Allied Sci. 2023;12:5582–5587. Doi: 10.55522/jmpas.V12I1.4232. DOI: https://doi.org/10.55522/jmpas.V12I1.4232
15.Maharani M, Lajuna L, Yuniwati C, Sabrida O, Sutrisno S. Phytochemical characteristics from Phaleria macrocarpa and its inhibitory activity on the peritoneal damage of endometriosis. J Ayurveda Integr Med. 2021;12(2):229–233. Doi: 10.1016/j.jaim.2020.06.002. DOI: https://doi.org/10.1016/j.jaim.2020.06.002
16.Maharani M, Sutrisno S. Phaleria macrocarpa Flavonoid as a Potent MMP-1 Inhibitor for Endometriosis Therapy: In silico Study. Asian J Health Res. 2022;1(2):7–11. Doi: 10.55561/ajhr.v1i2.24. DOI: https://doi.org/10.55561/ajhr.v1i2.24
17.Irwanto Y, Wiyono T, Wardani K. The Effect of Flavonoid Extract from Phaleria macrocarpa to Proliferating Factors (MMP-1, MMP-3, MMP-7) in Endometriosis Mice Model. Asian J Health Res. 2023;2(3):25–30. Doi: 10.55561/ajhr.v2i3.102. DOI: https://doi.org/10.55561/ajhr.v2i3.102
18.Febriani A, Sutrisno S, Irwanto Y, Baihaqi I, Wiyasa IWA, Rahardjo B. Effect of Phaleria macrocarpa Extract on NF-KB, MMP-2, and MMP-9 Expression in Endometriosis Mice Model. Asian J Health Res. 2022;1(3):4-10. Doi: 10.55561/ajhr.v1i3.35. DOI: https://doi.org/10.55561/ajhr.v1i3.35
19.Sutrisno S, Maharani M. Genistein Ameliorated Vascular Endothelial Growth Factor-A (VEGF-A) and Estrogen Receptor-Alpha (ER-α) in Endometriosis Mice Model, In Vivo and In Silico. Sci World J 2024;2024(1):5338212. Doi: 10.1155/2024/5338212. DOI: https://doi.org/10.1155/2024/5338212
20.Sutrisno S, Ratsamanda HB, Wiryasa IWA, Susianto SC, Kusuma BM. Effect of Administration of Red Fruit (Pandanus conoideus L.) Extract in TNF-α, Microvessels Density Expression and Endometriosis Implant Area in Endometriosis Mice Model. Asian Journal of Health Research. 2022;1(2):25-28. Doi: 10.55561/ajhr.v1i2.3. DOI: https://doi.org/10.55561/ajhr.v1i2.3
21.Duarte S, Kato H, Kuriyama N, Suko K, Ishikawa T, Busuttil RW, Herschman HR, Coito AJ. Hepatic Ischemia and Reperfusion Injury in the Absence of Myeloid Cell-Derived COX-2 in Mice. PloS One. 2014;9(5):e96913. Doi: 10.1371/journal.pone.0096913. DOI: https://doi.org/10.1371/journal.pone.0096913
22.Djordjević G, Matusan-Ilijas K, Sinozić E, Damante G, Fabbro D, Grahovac B, Lucin K, Jonjić N. Relationship between vascular endothelial growth factor and nuclear factor-kappaB in renal cell tumors. Croatian Medical Journal. 2008;49(5):608-617. Doi: 10.3325/cmj.2008.5.608. DOI: https://doi.org/10.3325/cmj.2008.5.608
23.Mei J, Zhu XY, Jin LP, Duan ZL, Li DJ, Li MQ. Estrogen promotes the survival of human secretory phase endometrial stromal cells via CXCL12/CXCR4 up-regulation-mediated autophagy inhibition. Hum Reprod. 2015;30(7):1677–1689. Doi: 10.1093/humrep/dev100. DOI: https://doi.org/10.1093/humrep/dev100
24.Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen YM, Li HB. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients. 2016;8(8):515. Doi: 10.3390/nu8080515. DOI: https://doi.org/10.3390/nu8080515
25.Saito K, Matsuo Y, Imafuji H, Okubo T, Maeda Y, Sato T, Shamoto T, Tsuboi K, Morimoto M, Takahashi H, Ishiguro H, Takiguchi S. Xanthohumol inhibits angiogenesis by suppressing nuclear factor-κB activation in pancreatic cancer. Cancer Sci. 2018;109(1):132–140. Doi: 10.1111/cas.13441. DOI: https://doi.org/10.1111/cas.13441
26.Zhao W, Li Y, Cheng X, Wei H, Li P, Fan L, Liu K, Zhang S, Wang H. The antioxidant Glycitin protects against intervertebral disc degeneration through antagonizing inflammation and oxidative stress in nucleus pulposus cells. Aging. 2023;15(23):13693–13709. Doi: 10.18632/aging.205251. DOI: https://doi.org/10.18632/aging.205251
27.Liu X, Wang N, Fan S, Zheng X, Yang Y, Zhu Y, Lu Y, Chen Q, Zhou H, Zheng J. The citrus flavonoid naringenin confers protection in a murine endotoxaemia model through AMPK-ATF3-dependent negative regulation of the TLR4 signalling pathway. Sci Rep. 2016;6:39735. Doi: 10.1038/srep39735. DOI: https://doi.org/10.1038/srep39735
28.Hu LH, Liu JY, Yin JB. Eriodictyol attenuates TNBS-induced ulcerative colitis through repressing TLR4/NF-kB signaling pathway in rats. Kaohsiung J Med Sci. 2021;37(9):812–818. Doi: 10.1002/kjm2.12400. DOI: https://doi.org/10.1002/kjm2.12400
29.Salsano S, Pérez-Debén S, Quiñonero A, González-Martín R, Domínguez F. Phytoestrogen exposure alters endometrial stromal cells and interferes with decidualization signaling. Fertil Steril. 2019;112(5):947–958.e3. Doi: 10.1016/j.fertnstert.2019.06.014. DOI: https://doi.org/10.1016/j.fertnstert.2019.06.014
30.Lai ZZ, Yang HL, Ha SY, Chang KK, Mei J, Zhou WJ, Qiu XM, Wang XQ, Zhu R, Li DJ, Li MQ. Cyclooxygenase-2 in Endometriosis. Int J Biol Sci. 2019;15(13):2783–2797. Doi: 10.7150/ijbs.35128. DOI: https://doi.org/10.7150/ijbs.35128
31.Yang X, Tian Y, Liu J, Kou Y, Xie Y, Wang S, Zhao Y. Peony Pollen Protects against Primary Dysmenorrhea in Mice by Inhibiting Inflammatory Response and Regulating the COX2/PGE2 Pathway. Int J Mol Sci. 2023;24(24):17245. Doi: 10.3390/ijms242417245. DOI: https://doi.org/10.3390/ijms242417245
32.Bo C, Wang Y. Angiogenesis signaling in endometriosis: Molecules, diagnosis and treatment (Review). Mol Med Rep. 2024;29:43. Doi: 10.3892/mmr.2024.13167. DOI: https://doi.org/10.3892/mmr.2024.13167
33.Jin K, Qian C, Lin J, Liu B. Cyclooxygenase-2-Prostaglandin E2 pathway: A key player in tumor-associated immune cells. Front Oncol. 2023;13:1099811. Doi: 10.3389/fonc.2023.1099811. DOI: https://doi.org/10.3389/fonc.2023.1099811
34.Bourlev V, Volkov N, Pavlovitch S, Lets N, Larsson A, Olovsson M. The relationship between microvessel density, proliferative activity and expression of vascular endothelial growth factor-A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction. 2006;132(3):501–509. Doi: 10.1530/rep.1.01110. DOI: https://doi.org/10.1530/rep.1.01110
35.Ghalehbandi S, Yuzugulen J, Pranjol MZI, Pourgholami MH. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur J Pharmacol. 2023;949:175586. Doi: 10.1016/j.ejphar.2023.175586. DOI: https://doi.org/10.1016/j.ejphar.2023.175586
36.Sutrisno S, Aprina H, Simanungkalit HM, Andriyani A, Barlianto W, Sujuti H, Santoso S, Dwijayasa PM, Wahyuni ES, Mustofa E. Genistein modulates the estrogen receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. J Tradit Complement Med. 2018;8(2):278–281. Doi: 10.1016/j.jtcme.2017.03.002. DOI: https://doi.org/10.1016/j.jtcme.2017.03.002
37.Li Y, Adur MK, Kannan A, Davila J, Zhao Y, Nowak RA, Bagchi MK, Bagchi IC, Li Q. Progesterone Alleviates Endometriosis via Inhibition of Uterine Cell Proliferation, Inflammation and Angiogenesis in an Immunocompetent Mouse Model. PLoS One. 2016;11(10):e0165347. Doi: 10.1371/journal.pone.0165347. DOI: https://doi.org/10.1371/journal.pone.0165347
38.Kapoor R, Sirohi VK, Gupta K, Dwivedi A. Naringenin ameliorates progression of endometriosis by modulating Nrf2/Keap1/HO1 axis and inducing apoptosis in rats. J Nutr Biochem. 2019;70(2019):215–226. Doi: 10.1016/j.jnutbio.2019.05.003. DOI: https://doi.org/10.1016/j.jnutbio.2019.05.003
39.Mihai IT, Rudzitis-Auth J, Menger MD, Laschke MW. The Presence of Pre-Existing Endometriotic Lesions Promotes the Growth of New Lesions in the Peritoneal Cavity. Int J Mol Sci. 2023;24(18):13858. Doi: 10.3390/ijms241813858. DOI: https://doi.org/10.3390/ijms241813858
40.He Y, Wang J, Jiang X, Gao J, Cheng Y, Liang T, Zhou J, Sun L, Zhang G. Effects of an inhibitor of the SHH signaling pathway on endometrial cells of patients with endometriosis. BMC Mol Cell Biol. 2022;23(1):37. Doi: 10.1186/s12860-022-00426-5. DOI: https://doi.org/10.1186/s12860-022-00426-5
41.Driva TS, Schatz C, Haybaeck J. Endometriosis-Associated Ovarian Carcinomas: How PI3K/AKT/mTOR Pathway Affects Their Pathogenesis. Biomol. 2023;13(8):1253. Doi: 10.3390/biom13081253. DOI: https://doi.org/10.3390/biom13081253
42.Chopyak VV, Koval HD, Havrylyuk AM, Lishchuk-Yakymovych KA, Potomkina HA, Kurpisz MK. Immunopathogenesis of endometriosis - a novel look at an old problem. Cent Eur J Immunol. 2022;47(1):109–116. Doi: 10.5114/ceji.2022.113830. DOI: https://doi.org/10.5114/ceji.2022.113830