Characterization and Temperature-Dependent Adsorption Potential of Hydrothermally Synthesized Manganese Oxide Nanoparticles
Main Article Content
Abstract
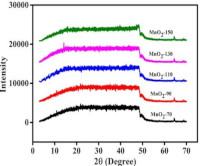
Water pollution usually results from discharge of untreated or partially treated human and industrial waste into water bodies, and nanomaterials are increasingly being recognized as a sustainable alternative remediation approach. The aim of the present study is to determine the structural properties and possible adsorption property of hydrothermally synthesized MnO2 nanoparticles. The synthesized nanoparticles were characterized using X-ray diffraction, scanning electron microscopy, energy-dispersive X-ray spectroscopy, Brunauer-Emmett-Teller surface area analysis, Fourier-transform infrared spectroscopy and UV-Visible spectroscopy. The nanoparticles primarily exhibited small crystallite sizes, structural disorder, and mostly amorphous material. Manganese was dominant in the nanoparticles as evidenced by surface shape and chemical composition but diminished with rising temperature. The surface area peaked at 110oC and decreased at higher temperatures possibly due to pore collapse and sintering effect, suggesting that annealing temperature had a significant impact on the mesoporous structure. FTIR and UV-Visible data suggest the presence of surface hydroxyl groups evidenced by moisture absorption, an O-Mn-O stretching that promotes hydrogen bonding and bandgap energies that allow for possible visible-light photocatalysis. Incorporating these results with existing research, the adsorption mechanism is expected to include electrostatic interactions controlled by surface charge, chemisorption via redox-active Mn sites, cation exchange regulated by potassium intercalation, and synergistic physisorption. The study also suggests that controlled thermal treatment enhanced adsorption performance as nanoparticles annealed at 90-110°C are expected to exhibit optimal adsorption qualities. Overall, findings from this study offer a starting point for producing efficient MnO2-based adsorbents capable of removing organic pollutants and heavy metals in aquatic environment.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Abdullah M, Alahmari SD, Alharbi FF, Ejaz SR, Waheed MS, Aman S, Al-Sehemi AG, Henaish AMA, Ahmad Z, Farid HMT. Facile synthesis of MnO2/g-C3N4 for photocatalytic reduction of methylene blue dye under visible light. J Mater Sci: Mater Electron. 2024; 35:517. https://doi.org/10.1007/s10854-024-12166-7
2.Dhanapal J, Saravanan NS, Jayaprakash NS, Sivakumar NV, Muniasamy NSK, Deivasigamani NV, Shanmugam NB, Sampathkumar NV. Eco-friendly concrete solutions: The role of titanium dioxide nanoparticles in enhancing durability and reducing environmental pollutants-A review. J Environ Nanotechnol. 2024;13(3):332-344. Doi: 10.13074/jent.2024.09.243894
3.Nagal V, Tuba T, Kumar V, Alam S, Ahmad A, Hafiz AK, Alshammari MB, Ahmad R. A non-enzymatic electrochemical sensor composed of nano-berries shaped cobalt oxide nanostructures on a glassy carbon electrode for uric acid detection. New J Chem. 2022; 46(25): 12333-12341. https://doi.org/10.1039/d2nj01961b
4.Alharbi FF, Abdullah M, Aman S, Gouadria S, Sadaf A, Abdel T, Taha M, Farid HMT. Development of environmental friendly Mo-doped MnO2 via hydrothermal route for supercapacitor as pollution-free source of energy. Appl Phys A. 2024; 130:236. https://doi.org/10.1007/s00339-024-07359-0
5.Yetim NK. Hydrothermal synthesis of Co3O4 with different morphology: Investigation of magnetic and electrochemical properties. J Mol Struct. 2021; 1226:129414. https://doi.org/10.1016/j.molstruc.2020.129414.
6.Navrotsky A, Ma C, Lilova K, Birkner N. Nanophase transition metal oxides show large thermodynamically driven shifts in oxidation-reduction equilibria. Sci. 2010; 330:199-201. Doi:10.1126/science.1195875
7.Navrotsky A. Nanoscale effects on thermodynamics and phase equilibria in oxide systems. ChemPhysChem. 2011; 12(12): 2207-2215. https://doi.org/10.1002/cphc.201100129
8.Li F, Wang J, Liu L, Qu J, Li Y, Bandari VK, Karnaushenko D, Becker C, Faghih M, Kang T, Baunack S, Zhu M, Zhu F, Schmidt OG. Self-assembled flexible and integratable 3D microtubular asymmetric supercapacitors. Adv Sci. 2019; 6(20):1901051 https://doi.org/10.1002/advs.201901051
9.Luo S, Xie L, Han F, Wei W, Huang Y, Zhang H, Zhu M Schmidt OG, Wang L. Nanoscale parallel circuitry based on interpenetrating conductive assembly for flexible and high-power zinc ion battery. Adv Funct Mater. 2019; 29(28):1901336 https://doi.org/10.1002/adfm.201901336
10.Wei H, Wang X, Zhang D, Du W, Sun X, Jiang F, Shi T. Facile synthesis of lotus seedpod-based 3D hollow porous activated carbon/manganese dioxide composite for supercapacitor electrode. J Electroanal Chem. 2019; 853:113561. https://doi.org/10.1016/j.jelechem.2019.113561.
11.Dang TD, Le TTH, Hoang TBT, Mai TT. Synthesis of nanostructured manganese oxides based materials and application for supercapacitor. Adv Nat Sci Nanosci Nanotechnol. 2015; 6(2):025011. https://doi.org/10.1088/2043-6262/6/2/025011
12.Wei Z, Pashchenko AV., Liedienov N, Zatovsky IV., Butenko DS, Li Q, Fesich IV, Turchenko V, Zubov E, Polynchuk PY, Pogrebnyak VG, Poroshin VM, Levchenko GG. Multifunctionality of lanthanum-strontium manganite nanopowder. Phys Chem Chem Phys. 2020; 22(21):1426. https://doi.org/10.1039/d0cp01426e
13.Tuutijärvi T, Lu J, Sillanpää M, Chen G. Adsorption mechanism of arsenate on crystal γ-Fe2O3 nanoparticles. J Environ Eng. 2010; 136(9): 1943. https://doi.org/10.1061/(asce)ee.1943-7870.0000233
14.Sun M, Lan B, Lin T, Cheng G, Ye F, Yu L, Cheng X, Zheng X. Controlled synthesis of nanostructured manganese oxide: Crystalline evolution and catalytic activities. Cryst Eng Comm. 2013; 15: 7010-7018. https://doi.org/10.1039/c3ce40603b
15.Annisa R, Lestari RI, Aqila SS, Fanany CT, Fitrianingsih AA, Rachmawati E, Rahmadiani N, Muti’ah R. Optimisation and characterisation of Dayak onion (Eleutherine palmifolia (L.) Merr) extract nanoparticles using cross-link method. Trop J Nat Prod Res. 2025; 9(2): 480-486. https://doi.org/10.26538/tjnpr/v9i2.10
16.Friday MD. Green synthesis, characterization and applications of manganese oxide nanoparticles. NanoEra. 2025; 5(1): 28-41. https://doi.org/10.5281/zenodo.15729170
17.Akinniyi JN. Synthesis and characterization of copper nanoparticles using Allium cepa (L.) outer peel at ambient temperature. Trop J Nat Prod Res. 2025; 9(3): 1144-1149. https://doi.org/10.26538/tjnpr/v9i3.32
18.Siddique MAB, Bithi UH, Ahmed AN, Gafur MA, Reaz AH, Roy CK, Islam MM, Firoz SH. Preparation of manganese oxide nanoparticles with enhanced capacitive properties utilizing gel formation method. ACS Omega. 2022; 7(51): 5872. Doi:10.1021/acsomega.2c05872
19.Li M, Kuang S, Kang Y, Ma H, Dong J, Guo Z. Recent advances in application of iron-manganese oxide nanomaterials for removal of heavy metals in the aquatic environment. Sci Total Environ. 2022; 819: 153157. https://doi.org/10.1016/j.scitotenv.2022.153157
20.Gan YX, Jayatissa AH, Yu Z, Chen X, Li M. Hydrothermal synthesis of nanomaterials. J Nanomater. 2020; 8917013. https://doi.org/10.1155/2020/8917013
21.Schmidt R, Prado-Gonjal J, Moran E. Microwave assisted hydrothermal synthesis of nanoparticles. Appl Phys. 2022; 561-572. https://doi.org/10.48550/arxiv.2203.02394
22.Raheem ZH, Al Sammarraie AMA. Synthesis of different manganese dioxide nanostructures and studding the enhancement of their electrochemical behavior in zinc-MnO2 rechargeable batteries by doping with copper. AIP Conf Proc. 2020; 2213(1): 020187. https://doi.org/10.1063/5.0000246
23.Augustin M, Fenske D, Bardenhagen I, Westphal A, Knipper M, Plaggenborg T, Kolny-Olesiak J, Parisi J. Manganese oxide phases and morphologies: A study on calcination temperature and atmospheric dependence. Beilstein J Nanotechnol. 2015; 6(1): 47-59. https://doi.org/10.3762/bjnano.6.6
24.Droepenu EK, Wee BS, Chin SF, Kok KY, Maligan MF. Zinc oxide nanoparticles synthesis methods and its effect on morphology: A review. Biointerface Res Appl Chem. 2022; 12(3): 4261-4292. https://doi.org/10.33263/briac123.42614292
25.Zhao C, Wang B, Theng BKG, Wu P, Liu F, Wang S, Lee X, Chen M, Li L, Zhang X. Formation and mechanisms of nano-metal oxide-biochar composites for pollutants removal: A review. Sci Total Environ. 2021; 767:145305. https://doi.org/10.1016/j.scitotenv.2021.145305
26.Thilaga-Sundari D, Silambarasan D, Sarika R. Electrochemical performance of manganese dioxide (MnO₂) nanoparticles synthesized by co-precipitation method. Nanotechnol Perceptions. 2024; 20(7):3869-3878.
27.Wang R, Gao P, Yuan S, Li Y, Liu Y, Huang C. Precise regulation of the phase transformation for pyrolusite during the reduction roasting process. Int J Miner Metall Mater. 2024; 31:81-90. https://doi.org/10.1007/s12613-023-2688-4
28.Kumar N, Thorat ST, Reddy KS. Multi biomarker approach to assess manganese and manganese nanoparticles toxicity in Pangasianodon hypophthalmus. Sci Rep. 2023; 13:8505. https://doi.org/10.1038/s41598-023-35787-0
29.Tang SF, Zhou H, Tan WT, Huang JG, Zeng P, Gu JF, Liao B-H. Adsorption characteristics and mechanisms of Fe-Mn oxide modified biochar for Pb(II) in wastewater. Int J Environ Res Public Health. 2022;19(14):8420. https://doi.org/10.3390/ijerph19148420
30.Xie M, Zhang X, Wang R, Jiao Y, Shu Z, Shan S, Bian Y, Lin H, Chen J, Xu Y. Mn-O bond engineering mitigating Jahn-Teller effects of manganese oxide for aqueous zinc-ion battery applications. Chem Eng J. 2024; 494:152908. https://doi.org/10.1016/j.cej.2024.152908
31.Namdari T, Astinchap B, Moradian R. Effect of annealing temperature on optical properties, microstructure and surface morphology of manganese dioxide layers. Optoelectron Adv Mater Rapid Commun. 2023; 17(3-4):152-158.
32.Wen J, Fang Y, Zeng G. Progress and prospect of adsorptive removal of heavy metal ions from aqueous solution using metal–organic frameworks: A review of studies from the last decade. Chemosphere. 2018; 201:627-643. https://doi.org/10.1016/j.chemosphere.2018.03.047
33.Xiao L, Deng Y, Zhou H, Lu F, Ke C, Ye Y, Pei X, Xia D, Pan F. Activated carbon fiber mediates efficient activation of peroxymonosulfate systems: Modulation of manganese oxides and cycling of manganese species. Chinese Chem Lett. 2023; 34(12):108407. https://doi.org/10.1016/j.cclet.2023.108407.
34.Falahatgar SS, Ghodsi FE. Annealing temperature effects on the optical properties of MnO2: Cu nanostructured thin films. Int J Nanosci Nanotechnol. 2016; 12(1):7-18.
35.Yao Y, Xu C, Yu S, Zhang D, Wang S. Facile synthesis of Mn3O4 reduced graphene oxide hybrids for catalytic decomposition of aqueous organics. Ind Eng Chem Res. 2013; 52(10):3637-3645. Doi:10.1021/ie303220x
36.Dessie Y, Tadesse S, Eswaramoorthy R. Physicochemical parameter influences and their optimization on the biosynthesis of MnO2 nanoparticles using Vernonia amygdalina leaf extract. Arab J Chem. 2020; 13(8):6472-6492. https://doi.org/10.1016/j.arabjc.2020.06.006
37.Mekuria AT. Biosynthesis of manganese dioxide nanoparticles and optimization of reaction variables. J Nanotechnol Nanomater. 2024; 5(1):31-45.
38.Hassan A, Haris M, Ullah Khan S, Khan I, Akif M, Akhtar N. Lathyrus aphaca extract MnO nanoparticles: Synthesis, characterization, and photocatalytic degradation of methylene blue dye. Photocatal Res Potential. 2024;1(3):10004. https://doi.org/10.35534/prp.2024.10004
39.Zhang H, Zhang Y, Pan Y, Wang F, Sun Y, Wang S, Wang Z, Wu A, Zhang Y. Efficient removal of heavy metal ions from wastewater and fixation of heavy metals in soil by manganese dioxide nanosorbents with tailored hollow mesoporous structure. Chem Eng J. 2023; 459:141583. https://doi.org/10.1016/j.cej.2023.141583
40.Asghar HMA, Hussain SN, Brown NW, Roberts EPL. Comparative adsorption–regeneration performance for newly developed carbonaceous adsorbent. J Ind Eng Chem. 2019; 69:90-98. https://doi.org/10.1016/j.jiec.2018.09.012
41.Liu X, Kifle MT, Xie H, Xu L, Luo M, Li Y, Huang Z, Gong Y, Wu Y, Xie C. Biomineralized manganese oxide nanoparticles synergistically relieve tumor hypoxia and activate immune response with radiotherapy in non-small cell lung cancer. Nanomater. 2022; 12(18):3138. Doi:10.3390/nano12183138.
42.Pal Singh J, Kumar M, Sharma A, Pandey G, Chae KH, Lee S. Bottom-up and Top-down approaches for MgO. Sonochemical Reactions. IntechOpen; 2020. http://dx.doi.org/10.5772/intechopen.91182
43.Manousi N, Giannakoudakis DA, Rosenberg E, Zachariadis GA. Extraction of metal ions with metal–organic frameworks. Mol. 2019;24(24):1-21. https://doi.org/10.3390/molecules24244605
44.Vyas S. Carbonaceous nanomaterials for water pollution remediation: An overview. EPRA Int J Multidiscip Res. 2021; 7(1):6185. https://doi.org/10.36713/epra6185
45.Ali S, Zahra H, Ahmad MU, Abdel-Rheem AA, Afzaal M, Nawaz R, Abbasi BBK, Irfan A, Jardan YAB. Synergistic photocatalytic and biomedical applications of Ag₂O-immobilized Bacillus subtilis-hyaluronic acid. Microb Cell Fact. 2025; 24:129. https://doi.org/10.1186/s12934-025-02750-9
46.Chenab KK, Sohrabi B, Esrafili M. pH-sensitive organic diimide materials-based superhydrophobic surface for oil-water separation applications. Mater Res Express. 2019; 6(12): 125112. Doi:10.1088/2053-1591/ab657b
47.Sousa Neto, VDO, Freire TM, Saraiva GD, Muniz CR, Cunha MS, Fechine PBA, Nascimento RFD. Water treatment devices based on zero-valent metal and metal oxide nanomaterials. In: Nascimento RFD, Ferreira OP, De Paula AJ, Sousa Neto VDO (Eds.). Nanomaterials applications for environmental matrices. Elsevier; 2019. 187-225 p. https://doi.org/10.1016/B978-0-12-814829-7.00005-7.