Potential Cytotoxic Isolates of Spigelia anthelmia Linn. against T47D and WiDr Cancer Cells
Main Article Content
Abstract
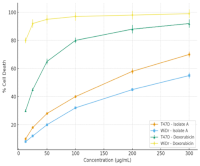
Natural products continue to provide valuable leads for anticancer drug discovery, yet limited studies have addressed the bioactive constituents of Spigelia anthelmia Linn. This research aimed to isolate and characterize cytotoxic metabolites of S. anthelmia using a bioassay-guided isolation approach. Sequential extraction, vacuum liquid chromatography (VLC), and preparative thin-layer chromatography (PTLC) yielded a semi-purified fraction designated as Isolate A. Cytotoxicity was evaluated against T47D breast cancer and WiDr colon cancer cells using the MTT assay. Isolate A significantly reduced cell viability in a dose-dependent manner (ANOVA, p < 0.05), with IC₅₀ values of 166.92 ± 5.10 µg/mL (T47D) and 245.24 ± 6.25 µg/mL (WiDr), whereas doxorubicin exhibited IC₅₀ values of 40.05 ± 2.30 µg/mL and 3.57 ± 0.45 µg/mL, respectively. Flow cytometry with Annexin V–FITC/PI staining revealed a visible shift of cell populations toward early and late apoptotic quadrants, indicating apoptosis as the predominant mechanism of cell death. Spectroscopic characterization by FTIR, LC–MS, and NMR suggested that Isolate A is a phenolic/alkaloid-type compound (m/z 218 [M+H]⁺) containing hydroxyl, carbonyl, and heteroaromatic groups. Collectively, these findings demonstrate that S. anthelmia contains apoptosis-inducing metabolites with measurable cytotoxicity, supporting its potential as a natural source of anticancer leads and warranting further purification and mechanistic investigation. To our knowledge, this is the first study to combine bioassay-guided isolation, apoptosis profiling, and spectroscopic characterisation of S. anthelmia metabolites in T47D and WiDr cancer cells.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Corsello SM, Nagari RT, Spangler RD, Rossen J, Kocak M, Bryan JG. Discovering the anticancer potential of non-oncology drugs by systematic viability profiling. Nat Cancer. 2020;1(2):235–248. Doi: 10.1038/s43018-019-0018-6.
2. Gezici S, Şekeroğlu N. Current perspectives in the application of medicinal plants against cancer: novel therapeutic agents. Anticancer Agents Med Chem. 2019;19(1):101–111. Doi: 10.2174/1871520619666181224121004.
3. Erb M, Kliebenstein DJ. Plant secondary metabolites as defences, regulators and primary metabolites: the blurred functional trichotomy. Plant Physiol. 2020;184(1):39–52. Doi: 10.1104/pp.20.00433.
4. Pfeffer CM, Singh ATK. Apoptosis: a target for anticancer therapy. Int J Mol Sci. 2018;19(2):448. Doi: 10.3390/ijms19020448.
5. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021; 71(3):209–249. Doi: 10.3322/caac.21660.
6. Huynh QDT, Phan TTT, Liu TW, Duong TLT, Hsu SJ, Kuo CC, Chu MH, Wang YH, Nguyen TV, Shen YA, Fan YJ, Nguyen DK, Vo TH, Lee CK. Cytotoxicity-guided isolation of elatostemanosides I–VI from Elatostema tenuicaudatum W.T. Wang and their cytotoxic activities. RSC Adv. 2025; 15(14):10639–10652. Doi: 10.1039/D4RA09007A.
7. Yang J, Hu DB, Xia MY, Luo JF, Li XY, Wang YH. Bioassay-guided isolation of cytotoxic constituents from the flowers of Aquilaria sinensis. Nat Prod Bioprospect. 2022;12(1):11. Doi: 10.1007/s13659-022-00334-3.
8. Chen L, Liu Y, Li Y, Yin W, Cheng Y. Anti-cancer effect of sesquiterpene and triterpenoids from agarwood of Aquilaria sinensis. Molecules. 2022;27(16):5350. Doi: 10.3390/molecules27165350.
9. Van Wyk AS, Prinsloo G. Health, safety and quality concerns of plant-based traditional medicines and herbal remedies: a review. S Afr J Bot. 2020;133:54–62. Doi: 10.1016/j.sajb.2020.06.017.
10. Nelson LS, Shih RD, Balick MJ, Lampe KF. Handbook of Poisonous and Injurious Plants. 2nd ed. New York: Springer; 2007. p. 21-34.
11. Akbar S. Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications. Springer; 2020.
12. Raszewski JA, Sharma S. Physiology, ryanodine receptor. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
13. Taupik M, Suryadi AMA, La Kilo J, Uno WZ, Badjeber SB. Identification of secondary metabolite compounds in leaves of Spigelia anthelmia L. and antioxidant activity using the DPPH (1,1-diphenyl-2-picrylhydrazyl) method. J Syifa Sci Clin Res. 2022;4(3):694–708. Doi:10.37311/jsscr.v4i3.15927.
14. Danlami U, Cecilia OE, Ifeanyi OM. Evaluation of the phytochemicals and antimicrobial activities of the ethanolic, hexane and ethyl acetate extracts of Spigelia anthelmia leaves. Int J Pharm Chem. 2017;3(3):29–35.
15. Ukwade CE, Ebuehi OAT, Adisa RA. Phytochemical and cytotoxic screening of selected medicinal plants (Byrsocarpus coccineus, Terminalia avicennioides and Anogeissus leiocarpus) using brine shrimp (Artemia salina) lethality assay. Eur J Nutr Food Saf. 2020;12(4):60–71.
16. Assis LM, Bevilaqua CML, Morais SM, Vieira LS, Costa CTC, Souza JAL. Ovicidal and larvicidal activity in vitro of Spigelia anthelmia Linn. extracts on Haemonchus contortus. Vet Parasitol. 2003;117(1):43–49.
17. Ribeiro WLC, Andre WPP, Cavalcante GS, de Araújo-Filho JV, Santos JML, Macedo ITF, de Melo JV, de Morais SM, Bevilaqua CML. Effects of Spigelia anthelmia decoction on sheep gastrointestinal nematodes. Small Rumin. Res. 2017; 153:146–152. Doi: 10.1016/j.smallrumres.2017.06.001.
18. Chibuye B, Indra SS, Luke C, Kakoma MK. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci Afr. 2023;19:e01585. Doi:10.1016/j.sciaf.2022.e01585.
19. Coll JC, Bowden BF. The application of vacuum liquid chromatography to the separation of terpene mixtures. J Nat Prod. 1986;49(5):934–936.
20. Sonam M, Singh RP, Pooja S. Phytochemical screening and TLC profiling of various extracts of Reinwardtia indica. Int J Pharmacogn Phytochem Res. 2017;9(4):523–527.
21. Arung ET, Wicaksono BD, Handoko YA, Kusuma IW, Yulia D, Sandra F. Anti-cancer properties of diethyl ether extract of wood from sukun (Artocarpus altilis) in human breast cancer (T47D) cells. Trop J Pharm Res. 2009;8(4):317–324.
22. Sarker SD, Nahar L. An introduction to natural products isolation. In: Sarker SD, Nahar L, editors. Natural Products Isolation. Totowa (NJ): Humana Press; 2012. p. 1–25.
23. Wagner H, Bauer R, Melchart D, Xiao PG, Staudinger A, editors. Chromatographic Fingerprint Analysis of Herbal Medicines. Vol. III: Thin-Layer and High-Performance Liquid Chromatography of Chinese Drugs. Cham: Springer; 2015.
24. Ogbole OO, Ndabai NC, Akinleye TE, Attah AF. Evaluation of peptide-rich root extracts of Calliandra portoricensis (Jacq.) Benth (Mimosaceae) for in vitro antimicrobial activity and brine shrimp lethality. BMC Complement Med Ther. 2020;20(1):1–7. Doi: 10.1186/s12906-020-2836-6.
25. Wulandari DD, Risthanti RR, Sari EAP, Anisa H, Filia S. Phytochemical screening and toxicological evaluation using brine shrimp lethality test of ethanolic extract of Morinda citrifolia L. Bali Med J. 2022;11(2):561–565. Doi: 10.15562/bmj.v11i2.3119.
26. Hayaza S, Wahyuningsih SPA, Susilo RJK, Permanasari AA, Husen SA, Winarni D. Anticancer activity of okra raw polysaccharides extracts against human liver cancer cells. Trop J Pharm Res. 2019;18(8):1667–1672.
27. Ifora I, Hamidi D, Susanti M, Wahyuni FS. Dual-method apoptosis evaluation reveals the therapeutic potential of Garcinia cowa extract in combination with doxorubicin for breast cancer treatment. Trop J Nat Prod Res. 2025;9(7):3076–3081. Doi:10.26538/tjnpr/v9i7.18.
28. Alshehade SA, Almoustafa HA, Alshawsh MA, Chik Z. Flow cytometry-based quantitative analysis of cellular protein expression in apoptosis subpopulations: a protocol. Heliyon. 2024;10(13):e33665. Doi: 10.1016/j.heliyon.2024.e33665.
29. Cocan I, Alexa E, Danciu C, Radulov I, Galuscan A, Obistioiu D, Morvay AA, Sumalan RM, Poiana MA, Pop G, Dehelean CA. Phytochemical screening and biological activity of Lamiaceae family plant extracts. Exp Ther Med. 2018; 15(2):1863–1870. Doi: 10.3892/etm.2017.5640.
30. Rabel F, Sherma J. Review of the state of the art of preparative thin-layer chromatography. J Liq Chromatogr Relat Technol. 2017;40(4):165–176.
31. Mahmoud AM, Hernández Bautista RJ, Sandhu MA, Hussein OE. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid Med Cell Longev. 2019;2019:1–19.
32. Miranda CAN, Souza ATB de, Soares AKM da C, Bernardes-Oliveira E, Rocha HAO, Barbosa EG. Apoptosis and G2/M Phase Cell Cycle Arrest Induced by Alkaloid Erythraline Isolated from Erythrina velutina in SiHa Cervical Cancer Cell. Int J Mol Sci. 2025;26(10):4627. Doi: 10.3390/ijms26104627.
33. Al-Hayali MZ, Nge CE, Lim KH, Collins HM, Kam TS, Bradshaw TD. Conofolidine: a natural plant alkaloid that causes apoptosis and senescence in cancer cells. Molecules. 2024;29(11):2654.
34. Calaf GM, Crispin LA, Quisbert-Valenzuela EO. Noscapine and apoptosis in breast and other cancers. Int J Mol Sci. 2024;25(6):3536.
35. Ghisalberti EL. Detection and isolation of bioactive natural products. In: Atta-ur-Rahman, editor. Bioactive Natural Products. Boca Raton (FL): CRC Press; 2007. p. 25–90.