Enzyme-assisted Extraction Optimization of Bioactive Phenolic Components from Phyllanthus urinaria
Main Article Content
Abstract
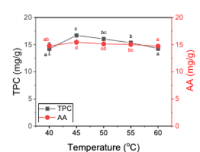
Phyllanthus urinaria is a traditional medicinal plant commonly used in Asian countries due to its rich phenolic content and antioxidant potential. The present study aimed to optimize the extraction method of polyphenols from P. urinaria using cellulase-assisted extraction (EAE). Key extraction parameters, including material-to-solvent ratio, extraction time, temperature, pH, and enzyme concentration, were first investigated through single-factor experiments to evaluate polyphenol recovery and antioxidant activity. Response Surface Methodology (RSM) was then employed to determine the optimal conditions, which were established at a solvent-to-material ratio of 1:19, an extraction time of 78 minutes, a temperature of 45.7°C, a pH of 5.02, and an enzyme concentration of 1.6%. Under these conditions, the extract exhibited a total polyphenol content of 17.20 mg/g and antioxidant activity of 15.51 mg/g by DPPH assay, while the quadratic RSM model showed strong predictive reliability (adjusted R² = 0.98; lack-of-fit, p > 0.05). HPLC-QTOF-MS analysis identified 10 major bioactive compounds, confirming the phytochemical richness of the extract. The findings highlight EAE as a sustainable and effective method for recovering functional constituents from P. urinaria, with promising applications in functional foods and pharmaceutical product development. Future studies should investigate the bioavailability and absorption mechanisms of the identified compounds to support their therapeutic utilization.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Tran TP, Nguyen TT, Tran GB. Anti-arthritis effect of ethanol extract of Sacha inchi (Plukenetia volubilis L.) leaves against complete Freund’s adjuvant-induced arthritis model in mice. Trop Life Sci Res. 2023; 34(3):237–257.
Liu L, Wang B, Ma Y, Sun K, Wang P, Li M, et al. A review of Phyllanthus urinaria L. in the treatment of liver disease: viral hepatitis, liver fibrosis/cirrhosis and hepatocellular carcinoma. Front Pharmacol. 2024; 15:1443667.
Geethangili M, Ding ST. A review of the phytochemistry and pharmacology of Phyllanthus urinaria L. Front Pharmacol. 2018; 9:1109.
Yuandani, Ilangkovan M, Jantan I, Mohamad HF, Husain K, Abdul Razak AF. Inhibitory effects of standardized extracts of Phyllanthus amarus and Phyllanthus urinaria and their marker compounds on phagocytic activity of human neutrophils. Evid Based Complement Alternat Med. 2013; 2013:603634.
Da Rocha CB, Norena CPZ. Microwave-assisted extraction and ultrasound-assisted extraction of bioactive compounds from grape pomace. Int J Food Eng. 2020; 16(1–2):20190191.
Dominguez-Rodriguez G, Marina ML, Plaza M. Enzyme-assisted extraction of bioactive non-extractable polyphenols from sweet cherry (Prunus avium L.) pomace. Food Chem. 2021; 339:128086.
Streimikyte P, Viskelis P, Viskelis J. Enzymes-assisted extraction of plants for sustainable and functional applications. Int J Mol Sci. 2022; 23(4):2359.
Okolie NP, Falodun A, Oluseyi D. Evaluation of the antioxidant activity of root extract of pepper fruit (Dennetia tripetala) and its potential for the inhibition of lipid peroxidation. Afr J Tradit Complement Altern Med. 2014; 11(3):221–227.
Balci M, Arikan-Abdulveli B, Yildiztugay E, Ozfidan-Konakci C. Role of syringic acid in enhancing growth, photosynthesis, and antioxidant defense in lettuce exposed to arsenic stress. Physiol Plant. 2025; 177(1):e70051.
Zain SNDM, Omar WAW. Antioxidant activity, total phenolic content and total flavonoid content of water and methanol extracts of Phyllanthus species from Malaysia. Pharmacogn J. 2018; 10(4):677–681.
Tai HP, Hong CTT, Huu TN, Thi TN. Extraction of custard apple (Annona squamosa L.) peel with supercritical CO₂ and ethanol as co-solvent. J Food Process Preserv. 2022; 46(11):e17040.
Thi TN, Tai HP. Microwave assisted extraction of custard apple (Annona squamosa L.) peel. Carpath J Food Sci Technol. 2023; 15(1):220–231.
Falodun A, Siraj R, Choudhary MI. GC-MS insecticidal leaf essential oil of P. staudtii Hutch and Dalz (Icacinaceae). Trop J Pharm Res. 2009; 8(2):139–143.
Gomez-Garcia R, Martinez-Avila GCG, Aguilar CN. Enzyme-assisted extraction of antioxidative phenolics from grape (Vitis vinifera L.) residues. 3 Biotech. 2012; 2:297–300.
Spigno G, Tramelli L, De Faveri DM. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J Food Eng. 2007; 81(1):200–208.
Puri M, Sharma D, Barrow CJ. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012; 30(1):37–44.
Park MK, Kim CH. Extraction of polyphenols from apple peel using cellulase and pectinase and estimation of antioxidant activity. J Korean Soc Food Sci Nutr. 2009; 38(5):535–540.
Antony A, Farid M. Effect of temperatures on polyphenols during extraction. Appl Sci. 2022; 12(4):2107.
Makebe CW, Desobgo ZSC, Ambindei WA, Billu A, Nso EJ, Nisha P. Optimization of pectinase-assisted extraction of Annona muricata L. juice and the effect of liquefaction on its pectin structure. J Sci Food Agric. 2020; 100(15):5487–5497.
Munde PJ, Muley AB, Ladole MR, Pawar AV, Talib MI, Parate VR. Optimization of pectinase-assisted and tri-solvent-mediated extraction and recovery of lycopene from waste tomato peels. 3 Biotech. 2017; 7(3):206.
Ruviaro AR, Barbosa PPM, Macedo GA. Enzyme-assisted biotransformation increases hesperetin content in citrus juice by-products. Food Res Int. 2019; 124:213–221.
Gani G, Naik HR, Jan N, Bashir O, Hussain SZ, Rather AH, et al. Physicochemical and antioxidant properties of pear juice prepared through pectinase enzyme-assisted extraction from William Bartlett variety. J Food Meas Charact. 2021; 15:743–757.
Pasquet PL, Julien-David D, Zhao M, Villain-Gambier M, Trebouet D. Stability and preservation of phenolic compounds and related antioxidant capacity from agro-food matrix: effect of pH and atmosphere. Food Biosci. 2024; 57:103586.
Heemann ACW, Heemann R, Kalegari P, Spier MR, Santin E. Enzyme-assisted extraction of polyphenols from green yerba mate. Braz J Food Technol. 2019; 22:e2017222.
Tang S, Ma Y, Dong X, Zhou H, He Y, Ren D, et al. Enzyme-assisted extraction of fucoidan from Kjellmaniella crassifolia based on kinetic study of enzymatic hydrolysis of algal cellulose. Algal Res. 2022; 66:102795.
Zheng HZ, Hwang IW, Chung SK. Enhancing polyphenol extraction from unripe apples by carbohydrate-hydrolyzing enzymes. J Zhejiang Univ Sci B. 2009; 10(12):912–919.
Henriksson G, Johansson G, Pettersson G. A critical review of cellobiose dehydrogenases. J Biotechnol. 2000; 78(2):93–113.
Raina D, Kumar V, Saran S. A critical review on exploitation of agro-industrial biomass as substrates for the therapeutic microbial enzymes production and implemented protein purification techniques. Chemosphere. 2022; 294:133712.
Ghandahari Yazdi AP, Barzegar M, Sahari MA, Ahmadi Gavlighi H. Optimization of the enzyme-assisted aqueous extraction of phenolic compounds from pistachio green hull. Food Sci Nutr. 2019; 7(1):356–366.
Myers RH, Montgomery DC, Vining GG, Borror CM, Kowalski SM. Response surface methodology: a retrospective and literature survey. J Qual Technol. 2004; 36(1):53–77.
Abdullah S, Pradhan RC, Pradhan D, Mishra S. Modeling and optimization of pectinase-assisted low-temperature extraction of cashew apple juice using artificial neural network coupled with genetic algorithm. Food Chem. 2021; 339:127862.
Aziz T, Qadir R, Anwar F, Naz S, Nazir N, Nabi G, Haiying C, Lin L, Alharbi M, Alasmari AF. Optimal enzyme-assisted extraction of phenolics from leaves of Pongamia pinnata via response surface methodology and artificial neural networking. Appl Biochem Biotechnol. 2024; 196(9):6508–6525.
Adeyi OE, Somade OT, Ajayi BO, James AS, Adeyi AO, Olayemi ZM, Tella NB. Syringic acid demonstrates better anti-apoptotic, anti-inflammatory and antioxidative effects than ascorbic acid via maintenance of the endogenous antioxidants and downregulation of pro-inflammatory and apoptotic markers in DMN-induced hepatotoxicity in rats. Biochem Biophys Rep. 2023; 33:101428.
Li T, Shen Y, Chen H, Xu Y, Wang D, Cui F, Han Y, Li J. Antibacterial properties of coaxial spinning membrane of methyl ferulate/zein and its preservation effect on sea bass. Foods. 2021; 10(10):2385.
Ramadan AMAA, Zidan SAH, Shehata RM, El-Sheikh HH, Ameen F, Stephenson SL, Al-Bedak OAM. Antioxidant, antibacterial, and molecular docking of methyl ferulate and oleic acid produced by Aspergillus pseudodeflectus AUMC 15761 utilizing wheat bran. Sci Rep. 2024; 14(1):3183.
Wang S, Zhang C, Yang G, Yang Y. Biological properties of 6-gingerol: a brief review. Nat Prod Commun. 2014; 9(7):1027–1032.
Henrion S, Mace A, Vallejos MM, Roisnel T, Carboni B, Villalgordo JM, Carreaux F. Asymmetric synthesis of trans-4,5-disubstituted γ-butyrolactones involving a key allylboration step: first access to (−)-nicotlactone B and (−)-galbacin. Org Biomol Chem. 2018; 16(10):1672–1678.