Antioxidant, Hepatoprotective and Toxicological Assessment of Dillenia ovata in Mice
DOI:
https://doi.org/10.26538/tjnpr/v9i10.32Keywords:
Dillenia ovata, Antioxidant Activity, Hepatoprotection, CCl₄–Induced Liver Injury, Medicinal HerbsAbstract
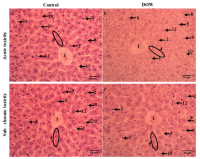
Dillenia ovata Wall. (DOW), collected from An Giang province, Vietnam, has been used traditionally in folk medicine. This study investigated the antioxidant and hepatoprotective potential of the crude ethanolic extract of Dillenia ovata. The antioxidant potential of the extract was evaluated in vitro using DPPH radical scavenging, reducing power (RP), and total antioxidant capacity (TAC) tests. Hepatoprotective effects were assessed in a Carbon tetrachloride (CCl₄)–induced liver injury model, where mice received Dillenia ovata extract at doses of 100, 200, or 400 mg/kg for 28 days. Biochemical analyses revealed that Dillenia ovata, especially at 400 mg/kg, significantly attenuated CCl₄–induced elevations in serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and malondialdehyde (MDA), while restoring glutathione (GSH) levels. Histopathological examination further confirmed the protective effect by showing improved hepatic architecture. In vivo safety evaluation showed no signs of toxicity in both acute and sub-chronic studies in mice. These findings suggest that Dillenia ovata extract exhibits potent antioxidant and hepatoprotective activities, highlighting its potential as a promising source of bioactive natural products.
References
1. Rui L. Energy Metabolism in the Liver. Physiol Behav. 2014;176(5):139–48.
2. Allameh A, Niayesh-Mehr R, Aliarab A, Sebastiani G, Pantopoulos K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants. 2023;12(9):1–23.
3. Conde de la Rosa L, Goicoechea L, Torres S, Garcia-Ruiz C, Fernandez-Checa JC. Role of Oxidative Stress in Liver Disorders. Livers. 2022;2(4):283–314.
4. Manh Dung Doan, San-Lang Wang, Van Bon Nguyen, Thi Kim Phung Phan, Tu Quy Phan, Tan Thanh Nguyen, Thi Huyen Nguyen, Quang Vinh Nguyen, Anh Dzung Nguyen.
Phytochemical profiles and novel biofunctions of Dillenia ovata Wall. ex Hook.f. et Thomson: A Vietnamese indigenous medicinal plant. Res Chem Intermed. 2023;(49) 5567–5593.
5. Anh VTT, Tran CL, Nguyen TN, Nguyen TLN, Vuong Thien Quy TQ, Trang ĐTX. Chemical composition and antimicrobial activity against Vibrio parahaemolyticus of Dillenia ovata extracts. Can Tho Univ J Sci. 2021;57(3):97–105.
6. Erin LSH, Mun PP, Ling NS, Ping OC, Jie SX, Ying NS. Evaluation of four extracts from Dilleniaovata stem bark and leaves for antibacterial and antifungal activity. Int J Pharm Pharm Sci. 2013;5(SUPPL 3):471–474.
7. Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules. 2022;27(4).
8. Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Japanese J Nutr Diet. 1986;44(6):307–315.
9. Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337–341.
10. OECD. Test No. 423: Acute Oral toxicity - Acute Toxic Class Method. 2002.
11. OECD. Repeated Dose 28-Day Oral Toxicity Study in Rodents (OECD TG 407). 2018. p. 477–90.
12. Kang H, Koppula S. Hepatoprotective effect of Houttuynia cordata thunb extract against carbon tetrachloride-induced hepatic damage in mice. Indian J Pharm Sci. 2014;76(4):267–273.
13. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Vol. 95, Analytical Biochemistry. 1979. p. 351–358.
14. Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Vol. 582, BBA - General Subjects. 1979. p. 67–78.
15. Meng X, Tang GY, Liu PH, Liu Q, Li H Bin, Zhao CJ. Antioxidant activity and hepatoprotective effect of 10 medicinal herbs on CCl4-induced liver injury in mice. World J Gastroenterol. 2020;26(37):5629–5645.
16. Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro Methods Evaluation of Antioxidant Activity. Saudi Pharm J. 2013;21(2):143–52. Doi: 10.1016/j.jsps.2012.05.002
17. Kun Qian, Shao Zhong, Keming Xie, Daozhan Yu, Rongze Yang DWG. Hepatic ALT isoenzymes are elevated in gluconeogenic conditions including diabetes and suppressed by insulin at the protein level. Diabetes Metab Res Rev. 2015;
18. Singh P, Khan S, Mittal ARK. Renal Function Test on the Basis of Serum Creatinine and Urea in Type-2 Diabetics and Nondiabetics. Vol. 3, Bali Medical Journal. 2014. p. 11.
19. Okon A, Jonathan N. Biochemical and Haematological Changes in Albino wistar Rats Following Administration of Eleophorbia drupifera Leaves Extract. Asian J Biochem Genet Mol Biol Vol. 2023;
20. Murray ATSAII. Histology, White Blood Cell Authors. Treasure Island (FL): StatPearls; 2021.
21. Islam Shawon S, Nargis Reyda R, Qais N. Medicinal Herbs and Their Metabolites with Biological Potential to Protect and Combat Liver Toxicity and Its Disorders: A review. Heliyon. 2024;10(3):e25340. Doi: 10.1016/j.heliyon.2024.e25340
22. Du Y, Zhang W, Qiu H, Xiao C, Shi J, Reid LM. Mouse Models of Liver Parenchyma Injuries and Regeneration. Front Cell Dev Biol. 2022;10(May):1–16.
23. Shao W, Xu H, Zeng K, Ye M, Pei R, Wang K. Advances in liver organoids: replicating hepatic complexity for toxicity assessment and disease modeling. Stem Cell Res Ther . 2025;16(1).
24. Thakur S, Kumar V, Das R, Sharma V, Mehta DK. Biomarkers of Hepatic Toxicity: An Overview. Curr Ther Res - Clin Exp. 2024;100:100737. Doi: 10.1016/j.curtheres.2024.100737
25. Behrendorff JBYH. Reductive Cytochrome P450 Reactions and Their Potential Role in Bioremediation. Front Microbiol. 2021;12(April):1–14.
26. Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014.
27. Kwon DH, Cha HJ, Lee H, Hong SH, Park C, Park SH. Protective effect of glutathione against oxidative stress-induced cytotoxicity in RAW 264.7 macrophages through activating the nuclear factor erythroid 2-related factor-2/heme oxygenase-1 pathway. Antioxidants. 2019;8(4).
28. Vairetti M, Di Pasqua LG, Cagna M, Richelmi P, Ferrigno A, Berardo C. Changes in glutathione content in liver diseases: An update. Antioxidants. 2021;10(3):1–39.
29. Mohamed FEZS, Tohamy HAS, El-Sakhawy M. Hepatoprotective activity of bio-fabricated carbon quantum dots-decorated zinc oxide against carbon tetrachloride-induced liver injury in male rats. BMC Pharmacol Toxicol . 2025;26(1).
Published
Issue
Section
License
Copyright (c) 2025 Tropical Journal of Natural Product Research

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.







