Formulation and Topical Safety Evaluation of Zanthoxylum rhetsa Spray Containing Essential Oil from Pericarp: A Phase 1, Double-blind, Non-Randomized, Single-Arm Patch Test Trial
DOI:
https://doi.org/10.26538/tjnpr/v9i10.31Keywords:
Zanthoxylum, Essential oils, Formulation, Dermal safety, Phase 1 clinical trial, Irritation, AllergyAbstract
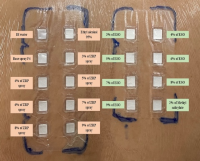
Our previous study demonstrated that essential oil from the pericarp of Zanthoxylum rhetsa (ESO) has a higher yield and greater potency in inhibiting prostaglandin E2 than essential oil from the fruit. This study aimed to formulate a topical spray containing ESO and to evaluate its safety through patch testing for irritation and allergy. A hydroalcoholic base spray (BS) was formulated and evaluated for appearance, skin feel, pH, viscosity, volume per spray, and evaporation time. Among BS F1–F5, F4 met all criteria and remained stable after six cooling– heating cycles. ESO was incorporated into F4 at 3–9% (v/v) to produce sprays, which passed stability tests. A non-randomized, double-blind, single-arm patch test was conducted in 12 healthy volunteers. Eighteen samples, including BS F4, spray products with 3–9% ESO, ESO alone at 3–9%, and controls, were applied to the upper back for 48 hours and evaluated at 30 minutes and 24 hours post-removal using the International Contact Dermatitis Research Group criteria. All concentrations showed acceptable characteristics; the 9% spray had optimal viscosity and slower evaporation. Clinically (10 completed), five showed no reaction at 30 minutes. One volunteer showed weak positive responses to 8% and 9% ESO and 2% methyl salicylate (score 0.42, slight irritation), and five showed angry back reactions. By 24 hours, all had resolved except one. Therefore, BS F4, sprays with 3–9% ESO, and ESO alone at 3–7% were safe, with the 9% formulation suitable for further clinical evaluation in pain management.
References
1. Aziz F, Rahman FI, Huque S, Ether SA, Hasan CM, Ahsan M. Zanthoxylum rhetsa (Roxb.) DC: A systemic review on traditional uses, phytochemistry, and pharmacology. J Pharm Pharmacogn Res. 2022; 10(1):52-72.
2. Imphat C, Thongdeeying P, Itharat A, Panthong S, Makchuchit S, Ooraikul B, Davies NM. Anti-inflammatory investigations of extracts of Zanthoxylum rhetsa. Evid Based Complement Alternat Med. 2021; 2021:5512961.
3. Imphat C, Woottisin N. Development of massage oil containing essential oil from Zanthoxylum limonella fruit and pain relief effect on calf muscle in healthy volunteers. J Thai Trad Alt Med. 2021; 19(1):147–160.
4. Yang D, Xu K, Xu X, Xu P. Revisiting prostaglandin E2: A promising therapeutic target for osteoarthritis. Clin Immunol. 2024; 260:109904.
5. Jiang H, Ji P, Shang X, Zhou Y. Connection between osteoarthritis and nitric oxide: From pathophysiology to therapeutic target. Molecules. 2023; 28(4):1683.
6. GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023; 5(9):e508–e522.
7. Leanpolchareanchai J, Teeranachaideekul V. Topical microemulsions: skin irritation potential and anti-inflammatory effects of herbal substances. Pharmaceuticals. 2023; 16(7):999.
8. Dedebas T, Ekici L, Sagdic O. Influence of heating on thermal stability of essential oils during storage. J Essent Oil-Bear Plants. 2022; 25(3):611-625.
9. Desu HR, Narang AS, Kumar V, Thoma LA, Mahato RI. Chapter 10 - Liquid dosage forms. In: Dash AK, Singh S (Eds.). Pharmaceutics (Second Edition). Academic Press; 2024. 271–318 p.
10. Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013; 88(6):879–888.
11. Tramontana M, Hansel K, Bianchi L, Sensini C, Malatesta N, Stingeni L. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023; 10:1184289.
12. Patel K, Nixon R. Irritant contact dermatitis – a review. Curr Dermatol Rep. 2022; 11(2):41–51.
13. Litchman G, Nair PA, Atwater AR, Bhutta BS. Contact dermatitis. Treasure Island (FL): StatPearls Publishing; 2023.
14. Panichayupakaranant P, Reanmongkol W. Evaluation of chemical stability and skin irritation of lawsone methyl ether in oral base. Pharm Biol. 2002; 40(6):429–432.
15. Saingam W, Chankana N, Madaka F, Sueree L, Homchuam S. Formulation development of topical film forming spray from Piper nigrum L. TJPS. 2018; 42(Suppl):219–222.
16. Dahlizar S, Futaki M, Okada A, Kadhum WR, Todo H, Sugibayashi K. Design of a topically applied gel spray formulation with ivermectin using a novel low molecular weight gelling agent, palmitoyl‑glycine‑histidine, to treat scabies. Chem Pharm Bull. 2018; 66(3):327–333.
17. Barnes TM, Mijaljica D, Townley JP, Spada F, Harrison IP. Vehicles for drug delivery and cosmetic moisturizers: review and comparison. Pharmaceutics. 2021; 13(12):2012.
18. Sakpal TV. Sample size estimation in clinical trial. Perspect Clin Res. 2010; 1(2):67–69.
19. Suwannarat W, Achariyakul M, Itharat A, Kiettinun S. A clinical study phase I on safety of Thai medicinal formula “Benjalokawichien (Ha-Rak)” and each plant component extract. TMJ. 2012; 12(4):767–776.
20. Garg V, Brod B, Gaspari AA. Patch testing: uses, systems, risks/benefits, and its role in managing the patient with contact dermatitis. Clin Dermatol. 2021; 39(4):580–590.
21. Russo F, Lazzeri L, Falcinelli F. Reading patch test through line-field confocal optical coherence tomography eyes. Dermatitis. 2024; 35(2):118-120.
22. An SM, Ham H, Choi EJ, Shin MK, An SS, Kim HO, Koh JS. Primary irritation index and safety zone of cosmetics: retrospective analysis of skin patch tests in 7440 Korean women during 12 years. Int J Cosmet Sci. 2014; 36(1):62–67.
23. National Center for Biotechnology Information. PubChem Compound Summary for CID 1030, Propylene Glycol. [Online]. 2025 [cited 2025 Jul 11]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Propylene-Glycol.
24. National Center for Biotechnology Information. PubChem Compound Summary for CID 753, Glycerol. [Online]. 2025 [cited 2025 Jul 11]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Glycerin.
25. Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006; 28(5):359–370.
26. Shafi PM, Jose B, Radhamani KT, Clery RA. Influence of pH on essential oil composition of Zanthoxylum rhetsa seeds obtained by steam distillation. Flavour Fragr J. 2006; 21(2):317–318.
27 Imphat C, Thongdeeying P, Itharat A, Panthong S, Makchuchit S. Proven inhibitory effect on lipopolysaccharides-induced nitric oxide production of Zanthoxylum rhetsa under accelerated condition and stability affecting factors. AMJAM. 2021; 21(2):134–144.
28. Umar AK, Butarbutar M, Sriwidodo S, Wathoni N. Film-forming sprays for topical drug delivery. Drug Des Devel Ther. 2020; 14:2909–2925.
29. Guo Y. Impact of solid-state characteristics to the physical stability of drug substance and drug product. In: Huynh-Ba K (Eds.). Handbook of Stability Testing in Pharmaceutical Development. New York: Springer; 2009. 241–261 p.
30. Melveger AJ, Huynh-Ba K. Critical regulatory requirements for a stability program. In: Huynh-Ba K (Eds.). Handbook of Stability Testing in Pharmaceutical Development. New York: Springer; 2009. 9–19 p.
31. Apmann K, Fulmer R, Soto A, Vafaei S. Thermal conductivity and viscosity: review and optimization of effects of nanoparticles. Materials. 2021; 14(5):1291.
32. Ratnah S, Salasa AM, Ramadhan DSF. Formulation, physical quality, and microbial contamination tests of anti-acne cream from longan (Euphoria longan [Lour. Steud.]) seed extract. Trop J Nat Prod Res. 2023; 7(7):3297–3305.
33. Lukic M, Pantelic I, Savic SD. Towards optimal pH of the skin and topical formulations: from the current state of the art to tailored products. Cosmetics. 2021; 8(3):69.
34. Blaak J, Staib P. The relation of pH and skin cleansing. Curr Probl Dermatol. 2018; 54:132–142.
35. Craighead DH, Alexander LM. Topical menthol increases cutaneous blood flow. Microvasc Res. 2016; 107:39–45.
36. Hopewell S, Chan AW, Collins GS, Hrobjartsson A, Moher D, Schulz KF, Tunn R, Aggarwal R, Berkwits M, Berlin JA, Bhandari N, Butcher NJ, Campbell MK, Chidebe RCW, Elbourne D, Farmer A, Fergusson DA, Golub RM, Goodman SN, Hoffmann TC, loannidis JPA, Kahan BC, Knowles RL, Lamb SE, Lewis S, Loder E, Offringa M, Ravaud P, Richards DP, Rockhold FW, Schriger DL, Siegfried NL, Staniszewska S, Taylor RS, Thabane L, Torgerson D, Vohra S, White IR, Boutron I. CONSORT 2025 statement: updated guideline for reporting randomized trials. Nat Med. 2025; 31(6):1776-1783.
37. Li B, Cheng Y, Tan Y, Wang F, Hu W, Wang X, Liu W, Krutmann J, Wang S, Zou Y. Analysis of factors influencing patch test reactions: results from a large-population-based study in Chinese. J Cosmet Dermatol. 2022; 21(5):2183–2188.
38. Sapra A, Malik A, Bhandari P. Vital sign assessment. Treasure Island (FL): StatPearls Publishing; 2023.
39. Maier LE, Maibach HI, O’Malley M. Irritant dermatitis. In: Krieger R (Eds.). Hayes' handbook of pesticide toxicology (Third Edition). Academic Press; 2010. 647–659 p.
40. Mitchell JC. The angry back syndrome: eczema creates eczema. Contact Dermatitis. 1975; 1(4):193–194.
41. Srichaipor N, Pongcharoen P, Kanokkangsadal P, Itharat A. Skin irritation and allergic testing of Thai herbal extracts (Ha-Rak with turmeric) in healthy volunteers. TMJ. 2020; 20(2):165–174.
42. Guo J, Hu X, Wang J, Yu B, Li J, Chen J, Nie X, Zheng Z, Wang S, Qin Q. Safety and efficacy of compound methyl salicylate liniment for topical pain: A multicenter real-world study in China. Front Pharmacol. 2022; 13:1015941.
Published
Issue
Section
License
Copyright (c) 2025 Tropical Journal of Natural Product Research

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.







