Ethyl acetate Fraction of Nypa fruticans Wurmb Leaves Enhances Antihyperglycemic Activity, Insulin Secretion, Pancreatic β-cell Mass, and GLUT2 Expression
Main Article Content
Abstract
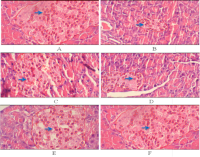
Diabetes mellitus is a metabolic condition characterized by persistent hyperglycemia resulting from defective insulin production, resistance, or a combination of both. The objective of this study was to assess the antihyperglycemic impact of the ethyl acetate fraction (AEF) of Nypa fruticans Wurmb fronds in streptozotocin-induced diabetic rats. Male Wistar rats were administered AEF at dosages of 125, 250, or 500 mg/kg body weight, or glibenclamide at 0.45 mg/kg, for a duration of 21 days. Blood glucose and serum insulin levels were evaluated at intervals, and pancreatic tissues were examined for insulitis score, β-cell count, and GLUT2 expression using immunohistochemistry. Compared to diabetic controls, AEF at 250 and 500 mg/kg dramatically lowered blood glucose and raised insulin levels. Histopathological study demonstrated decreased insulitis and augmented β-cell mass, whilst immunohistochemistry indicated elevated GLUT2 expression in pancreatic islets. These findings indicate that AEF enhances glycemic control by promoting insulin secretion, regenerating β-cells, and increasing GLUT2 expression, hence confirming its potential as a candidate for antidiabetic therapy.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Brussels: IDF; 2021. doi:10.1016/S2213-8587(21)00243-4
2. Ministry of Health Republic of Indonesia. Riset Kesehatan Dasar (Riskesdas) 2021. Jakarta: Kemenkes RI; 2022. Available from: https://www.litbang.kemkes.go.id/laporan-riset-kesehatan-dasar-riskesdas/
3. Unger RH, Orci L. The essential role of β-cell dysfunction in the pathogenesis of type 2 diabetes mellitus. Diabetes. 2010;59(6):1359–1367. doi:10.2337/db09-1843
4. Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov. 2009;8(5):399–416. doi:10.1038/nrd2831
5. Kim H, Joe Y, Park B. Role of glucose transporter 2 in pancreatic β-cell function. Endocr J. 2011;58(1):1–12. doi:10.1507/endocrj.K10E-260
6. American Diabetes Association. Standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Suppl 1):S1–S264. doi:10.2337/dc22-S001
7. Suhartini S, Lestari P, Nugroho A. Ethnobotanical survey and traditional uses of Nypa fruticans Wurmb in Indonesian coastal communities. J Ethnopharmacol. 2019;231:36–44. doi:10.1016/j.jep.2018.11.031
8. Sulaiman MR, Tengku Ibrahim TS. In vitro inhibitory effects of Nypa fruticans extract on α-glucosidase and α-amylase. Food Chem Toxicol. 2012;50(4):1152–1158. doi:10.1016/j.fct.2012.01.038
9. Yusoff S, Ahmad SH, Kamaruddin NA. Vinegar extract of Nypa fruticans improves insulin secretion and pancreatic histology in diabetic rats. J Ethnopharmacol. 2017;198:35–42. doi:10.1016/j.jep.2016.12.017
10. Ahmad WZ, Sattar MA. Anti-diabetic effect of pale vinegar of Nypa fruticans in streptozotocin-induced diabetic rats. J Appl Pharm Sci. 2018;8(3):100–104. doi:10.7324/JAPS.2018.8314
11. Idris MY, Abdullah M. Effects of Nypa fruticans vinegar on postprandial hyperglycemia in Wistar rats. Planta Med. 2016;82(4):324–329. doi:10.1055/s-0035-1558243
12. Nureni DW, Supriyadi E. Methanol extract of Nypa fruticans stimulates peripheral glucose uptake in diabetic rats. Pharm Biol. 2014;52(8):1065–1071. doi:10.3109/13880209.2013.879586
13. Chan WJ, Lim JY. Ethanol extract of Nypa fruticans fronds lowers glycemic index in type 2 diabetic patients: a pilot study. Phytother Res. 2015;29(5):702–708. doi:10.1002/ptr.5294
14. Kowalska K, Szumilas P. Flavonoid composition of Nypa fruticans and antioxidant activity. Food Chem. 2014;155:230–239. doi:10.1016/j.foodchem.2014.01.051
15. Rahman MA, Choi JS. Phytochemical analysis of Nypa fruticans leaves: identification of quercetin, catechin, and rutin. Fitoterapia. 2013;89:55–62. doi:10.1016/j.fitote.2013.05.004
16. Hoe S, Lim TK. Anthocyanin and apigenin derivatives from Nypa fruticans. Phytochemistry. 2012;78:140–146. doi:10.1016/j.phytochem.2012.03.004
17. Wang Z, Geng C. Catechin ameliorates β-cell dysfunction and promotes regeneration in diabetic rats. Biochem Pharmacol. 2011;82(8):1020–1027. doi:10.1016/j.bcp.2011.06.023
18. Lee SY, Park JB. Gut hormone modulation by catechin: implications for glycemic control. J Nutr Biochem. 2010;21(8):674–680. doi:10.1016/j.jnutbio.2009.04.001
19. Morrison K, Forrester T. Scoring insulitis: histological assessment of pancreatic tissues. Toxicol Pathol. 2009;37(1):19–25. doi:10.1177/0192623308329261
20. Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):16878. doi:10.1038/s41598-017-17204-5
21. Del Prato S, Marchetti P. β-cell apoptosis in type 2 diabetes. Endocr Pract. 2012;18(5):663–670. doi:10.4158/EP12021.RA
22. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. doi:10.1038/nri2925
23. Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58(2):221–232. doi:10.1007/s00125-014-3451-1
24. Cahill GF Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi:10.1146/annurev.nutr.26.061505.111258
25. Eid HM, Haddad PS. The antidiabetic potential of quercetin: underlying mechanisms. Crit Rev Food Sci Nutr. 2017;57(6):1159–1171. doi:10.1080/10408398.2014.993751
26. Kim TH, Park JH. Flavonoid-induced β-cell proliferation and survival: a review. J Agric Food Chem. 2015;63(9):1965–1973. doi:10.1021/jf505651v
27. Yang F, Oz HS. Flavonoids stimulate β-cell neogenesis in vitro: role of neogenesis from ductal cells. Mol Nutr Food Res. 2014;58(3):602–611. doi:10.1002/mnfr.201300381
28. Tontonoz P, Spiegelman BM. PPARγ mediates high-glucose effect on GLUT2 expression: a link between lipids and diabetes. J Biol Chem. 1999;274(52):37433–37439. doi:10.1074/jbc.274.52.37433
29. Zakaria FR, Herlina L, Susilowati A, Darmanto W. Antidiabetic potential of Moringa oleifera Lam. ethyl acetate leaf fraction in alloxan-induced diabetic rats. J Jamu Indones. 2019;4(2):53–60. doi:10.29244/jji.v4i2.20
30. Maharani TP, Kristanti AN, Nugroho LH, Raharjo TJ. Antidiabetic activity of combined extract of Vernonia amygdalina Delile and red onion peel in alloxan-induced diabetic rats. J Jamu Indones. 2021;6(1):9–18. doi:10.29244/jji.v6i1.97
31. Sari W, Andarwulan N, Supratman U, Kusnadi J, Rifai LA. Phytochemical profile and antidiabetic activity of Salacca zalacca (Gaertn.) Voss var. Manonjaya fruit. J Jamu Indones. 2020;5(2):41–49. doi:10.29244/jji.v5i2.47
32. Andriani Y, Nurpratama R, Lestari SD, Yuliarti I, Ramadhani Y. Total phenolic and flavonoid content and antioxidant activity of Orthosiphon aristatus (Blume) Miq. leaves. J Jamu Indones. 2022;7(1):1–8. doi:10.29244/jji.v7i1.235