Zingiber officinale Extract Inhibits Entry of SARS-CoV-2 D614G Virus-Like Particles to 6HBE14o- cells: In Vitro and In Silico Approaches
DOI:
https://doi.org/10.26538/tjnpr/v9i10.13Keywords:
Antivirus, D614G Mutation, SARS-CoV-2, Virus-like particle, Zingiber officinale, In silicoAbstract
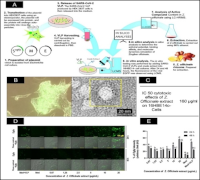
Global public health systems are facing challenges due to the COVID-19 pandemic, which is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Given increasing interest in plant-based antivirals, this study determined the potential of ethanol extract of red ginger (Zingiber officinale) to inhibit the attachment of SARS-CoV-2 virus-like particles (VLPs) to human bronchial epithelial cells. SARS-CoV-2 VLPs carrying the D614G spike mutation were generated in HEK293T cells using plasmids encoding the envelope (E), membrane (M), and spike-EGFP fusion proteins. EGFP was used to visualize and quantify VLP attachment. VLP formation was confirmed by fluorescence microscopy and transmission electron microscopy. Cells were exposed to 200 ng/mL of VLPs together with Z. officinale extract at 0.67 to 20 µg/mL for 24-48 h, and viral attachment was quantified via EGFP fluorescence intensity. For in silico analysis, identified compounds of Z. officinale extract were screened based on drug-likeness parameters for further processing to molecular docking and molecular dynamics simulations. The results showed that treatment with Z. officinale extract significantly reduced VLP attachment at concentrations of 0.67–10 µg/mL after 24 h. After 48 h, the lowest concentrations (0.67 and 1.25 µg/mL) maintained inhibitory effects from SARS-CoV-2 VLP, as indicated by reduced EGFP fluorescence. Of the 22 initially screened compounds, 9 met drug-likeness parameters. Among these, (2E)-3-(3,4-dimethoxyphenyl)prop-2-enoic acid and ferulic acid exhibited the strongest binding affinities to the SARS-CoV-2 spike protein (-5.3 and -5.5 kcal/mol, respectively). These findings suggest that Z. officinale contains bioactive compounds with potential as antiviral agents targeting SARS-CoV-2 variants.
References
1. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):1-9. doi: 10.1016/j.ijantimicag.2020.105924.
2. Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ. Coronavirus Disease 2019 (COVID-19): A perspective from China. Radiology. 2020;296(2):E15-E25. doi: 10.1148/radiol.2020200490.
3. Wang YT, Landeras-Bueno S, Hsieh LE, Terada Y, Kim K, Ley K, Shresta S, Saphire EO, Regla-Nava JA. Spiking pandemic potential: structural and immunological aspects of SARS-CoV-2. Trends Microbiol. 2020;28(8):605-618. doi: 10.1016/j.tim.2020.05.012.
4. Erharuyi O, Aghahowa S, Igbinosa E, Imieje VO, Ogbeide OK, Akhigbe IU, Nahandoo I, Olowoeyo BE, Akubuiro PC, Ayorinde J, Igoli JO, Falodun A. Anti-SARS-CoV-2 activity and acute toxicity screening of Annona muricata and Artemisia annua Leaf Extracts. Trop J Nat Prod Res. 2024;8(1):6028-6034. doi: 10.26538/tjnpr/v8i1.45.
5. Rizma BRP, Ananto AD, Sunarwidhi AL. The study of potential antiviral compounds from Indonesian medicinal plants as anti-COVID-19 with molecular docking approach. J Mol Docking. 2021;1(1):32–39. doi: 10.33084/jmd.v1i1.2307.
6. Zhan Y, Ta W, Tang W, Hua R, Wang J, Wang C, Lu W. Potential antiviral activity of isorhamnetin against SARS-CoV-2 spike pseudotyped virus in vitro. Drug Dev Res. 2021;82(8):1124-1130. doi: 10.1002/ddr.21815.
7. Dahham SS, Tabana YM, Iqbal MA, Ahamed MB, Ezzat MO, Majid AS, Majid AM. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules. 2015;20(7):11808-11829. doi: 10.3390/molecules200711808.
8. Ezez D, Tefera M. Effects of solvents on total phenolic content and antioxidant activity of ginger extracts. J Chem. 2021;2021(1):1-5. doi: 10.1155/2021/6635199.
9. Nur'aini AL, Hartati S, Untari T. In ovo inhibition of avian pox virus replication by mangosteen rind and red ginger ethanolic extracts. Vet World. 2021;14(10):2640-2645. doi: 10.14202/vetworld.2021.2640-2645.
10. Untari T, Widyarini S, Wibowo MH. Antiviral activity of the essential oil of red ginger on Avian Influenza. Vet. J. 2013;13(3):309–312.
11. Mignaqui AC, Ferella A, Cass B, Mukankurayija L, L'Abbé D, Bisson L, Sánchez C, Scian R, Cardillo SB, Durocher Y, Wigdorovitz A. Foot-and-Mouth Disease: Optimization, Reproducibility, and Scalability of High-Yield Production of Virus-Like Particles for a Next-Generation Vaccine. Front Vet Sci. 2020;7:1-9. doi: 10.3389/fvets.2020.00601.
12. Gourdelier M, Swain J, Arone C, Mouttou A, Bracquemond D, Merida P, Saffarian S, Lyonnais S, Favard C, Muriaux D. Optimized production and fluorescent labeling of SARS-CoV-2 virus-like particles. Sci Rep. 2022;12(1):1–15. doi: 10.1038/s41598-022-18681-z.
13. Le DT, Müller KM. In vitro assembly of virus-like particles and their applications. Life. 2021;11(4):1-18. doi: 10.3390/life11040334.
14. Korber B, Fischer WM, Gnanankaran S, Yoon H, Theiler J, Abfalterer W, Foley B, Giorgi EE, Bhattacharya T, Parker M, Partridge D, Evans C, de Silva TI, on behalf of the Sheffield COVID-19 Genomics Group, LaBranche CC, Montefiori DC. Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2. BioRxiv. 2020; 1–33. doi: 10.1016/j.cell.2020.06.043
15. Zhang S, Kou X, Zhao H, Mak KK, Balijepalli MK, Pichika MR. Zingiber officinale var. rubrum: red ginger's medicinal uses. Molecules. 2022;27(3):1-31. doi: 10.3390/molecules27030775.
16. Djati MS, Christina YI, Rifa'i M. The combination of Elephantopus scaber and Sauropus androgynus promotes erythroid lineages and modulates follicle-stimulating hormone and luteinizing hormone levels in pregnant mice infected with Escherichia coli. Vet World. 2021;14(5):1398-1404. doi: 10.14202/vetworld.2021.1398-1404.
17. Christina YI, Rifa'i M, Widodo N, Djati MS. Comparative study of antiproliferative activity in different plant parts of Phaleria macrocarpa and the underlying mechanism of action. Sci World J. 2022;2022:1-13. doi: 10.1155/2022/3992660.
18. Rohmah IN, Marlita M, Kusuma KH, Christina YI, Dwijayanti DR, Mustikaningtyas D, Widodo N, Djati MS. Corrigendum: Evaluating SARS-CoV-2 spike protein transfection in HEK-293T cells for VLP applications. J Exp Life Sci. 2025;15(2):45-51. doi: 10.21776/ub.jels.2025.015.02.01.
19. Christina YI, Nafisah W, Atho'illah MF, Rifa'i M, Widodo N, Djati MS. Anti-breast cancer potential activity of Phaleria macrocarpa (Scheff.) Boerl. leaf extract through in silico studies. J Pharm Pharmacogn Res. 2021;9(6):824–845. doi: 10.56499/jppres21.1092_9.6.824
20. Masruri M, Safitri A, Ulfa SM, Ferdian RF, Fabri ARA, Karta IW, Harrist M, Shalsadila AA. Phytochemical composition, antioxidant activity, and metabolite profiling of sequential extracts from Vitex trifolia leaves with Antibacterial potential: An integrated in vitro and in silico study. Trop J Nat Prod Res. 2025; 9(7):291-2991. doi: 10.26538/tjnpr/v9i7.5
21. Krieger E, Vriend G. New ways to boost molecular dynamics simulations. J Comput Chem. 2015;36(13):996-1007. doi: 10.1002/jcc.23899.
22. Suravajhala R, Parashar A, Choudhir G, Kumar A, Malik B, Nagaraj VA, Padmanaban G, Polavarapu R, Suravajhala P, Kishor PBK. Molecular docking and dynamics studies of curcumin with COVID-19 proteins. Netw Model Anal Health Inform Bioinform. 2021;10(1):1-44. doi: 10.1007/s13721-021-00312-8.
23. Deeba F, Malik MZ, Naqvi IH, Haider MSH, Shafat Z, Sinha P, Ishrat R, Ahmed A, Parveen S. Potential entry inhibitors of the envelope protein (E2) of Chikungunya virus: in silico structural modeling, docking and molecular dynamic studies. Virusdisease. 2017;28(1):39-49. doi: 10.1007/s13337-016-0356-2.
24. Chen H, Soroka DN, Hu Y, Chen X, Sang S. Characterization of thiol-conjugated metabolites of ginger
components shogaols in mouse and human urine and modulation of the glutathione levels in cancer cells by [6]-shogaol. Mol Nutr Food Res. 2013;57(3):447-458. doi: 10.1002/mnfr.201200679.
25. Sari D, Nasuha A. Nutrients content, phytochemical, and pharmacological activities of ginger (Zingiber officinale Rosc.): A review. Trop Biosci J Biol Sci 2021;1(2):11–18. doi: 10.32678/tropicalbiosci.v1i2.5246.
26. Kuo PC, Damu AG, Cherng CY, Jeng JF, Teng CM, Lee EJ, Wu TS. Isolation of a natural antioxidant, dehydrozingerone from Zingiber officinale and synthesis of its analogues for recognition of effective antioxidant and antityrosinase agents. Arch Pharm Res. 2005;28(5):518-528. doi: 10.1007/BF02977752.
27. Suherman M, Maulidya SAI. In silico study: secondary metabolites from red ginger rhizome (Zingiber officinale var. Rubrum) as potential inhibitors OF3CLpro and PLpro of SARS-CoV-2. Med Sains. 2023;8(3):1249–1262. doi: 10.37874/ms.v8i3.810.
28. Rao MJ, Tahir Ul Qamar M, Wang D, Ali Q, Ma L, Han S, Duan M, Hu L, Wang L. A high-throughput lipidomics and transcriptomic approach reveals novel compounds from sugarcane linked with promising therapeutic potential against COVID-19. Front Nutr. 2022;9:1-15. doi: 10.3389/fnut.2022.988249.
29. Katerina V, Klara U, Samnang N, Ladislav K. Chemical Composition of Essential Oils and Supercritical Carbon Dioxide Extracts from Amomum kravanh, Citrus hystrix and Piper nigrum 'Kampot'. Molecules. 2023;28(23):1-16. doi: 10.3390/molecules28237748.
30. Nakazawa T, Ohsawa K. Metabolism of [6]-gingerol in rats. Life Sci. 2002;70(18):2165-2175. doi: 10.1016/s0024-3205(01)01551-x.
31. Račková L, Cupáková M, Tažký A, Mičová J, Kolek E, Košt'álová D. Redox properties of ginger extracts: Perspectives of use of Zingiber officinale Rosc. as antidiabetic agent. Interdiscip Toxicol. 2013;6(1):26-33. doi: 10.2478/intox-2013-0005.
32. Liu H, Yang H, Zhao T, Lin C, Li Y, Zhang X, Ye Y, Liao J. Combined metabolome and transcriptome analyses of young, mature, and old rhizome tissues of Zingiber officinale Roscoe. Front Genet. 2021;12:1-16. doi: 10.3389/fgene.2021.795201.
33. Nievergelt A, Marazzi J, Schoop R, Altmann KH, Gertsch J. Ginger phenylpropanoids inhibit IL-1beta and prostanoid secretion and disrupt arachidonate-phospholipid remodeling by targeting phospholipases A2. J Immunol. 2011;187(8):4140-4150. doi: 10.4049/jimmunol.1100880.
34. Arias-Gaguancela O, Chapman KD. The biosynthesis and roles of n-acylethanolamines in plants. Adv Bot Res. 2022;101:345–373. doi: 10.1016/bs.abr.2021.07.002.
35. Ahmad B, Rehman MU, Amin I, Arif A, Rasool S, Bhat SA, Afzal I, Hussain I, Bilal S, Mir Mu. A Review on pharmacological properties of zzingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). Sci World J. 2015;2015:1-6. doi: 10.1155/2015/816364.
36. Saraç H, Demirbaş A, Tüzün B. Could Zingiber officinale plant be effective against omicron BA.2.75 of SARS-CoV-2?. Turk Comput Theor Chem. 2023;7(3):42–56. Doi: 10.33435/tcandtc.1198612
37. Swann H, Sharma A, Preece B, Peterson A, Eldredge C, Belnap DM, Vershinin M, Saffarian S. Author Correction: Minimal system for assembly of SARS-CoV-2 virus like particles. Sci Rep. 2021;11(1):9352. doi: 10.1038/s41598-021-88846-9.
38. Xu R, Shi M, Li J, Song P, Li N. Construction of SARS-CoV-2 Virus-Like Particles by mammalian expression system. Front Bioeng Biotechnol. 2020;8:1-6. doi: 10.3389/fbioe.2020.00862.
39. Halder SK, Sultana I, Shuvo MN, Shil A, Himel MK, Hasan MA, Shawan MMAK. In silico identification and analysis of potentially bioactive antiviral phytochemicals against SARS-CoV-2: A molecular docking and dynamics simulation approach. Biomed Res Int. 2023;2023:1-32. doi: 10.1155/2023/5469258.
Published
Issue
Section
License
Copyright (c) 2025 Tropical Journal of Natural Product Research

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.







