Cultivation of Native Tissue-Cultured Kappaphycus alvarezii Plantlets at Multiple Sites in The Myeik Archipelago, Myanmar
DOI:
https://doi.org/10.26538/tjnpr/v9i10.12Keywords:
Cottonii, Carrageenan, Kappaphycus alvarezii, Marine aquaculture, Myanmar Coastal Farming, Seaweed Cultivation, Tissue-Cultured PlantletsAbstract
Commercial cultivation of Kappaphycus alvarezii (Cottonii) offers opportunities for Myanmar's coastal economy through sustainable aquaculture and economic development. This study assessed the feasibility of large-scale K. alvarezii cultivation in the Myeik Archipelago using tissue-cultured plantlets. Growth performance was evaluated across three sites - Pyin Htet Aw, Thae Chaung, and Don Pale Aw - and the impact of environmental conditions on growth and productivity was examined. Tissue-cultured K. alvarezii plantlets were cultivated at three sites over five weeks. Growth performance and daily growth rate (DGR), along with environmental parameters including temperature and salinity, were monitored. Carrageenan yield and viscosity were measured to evaluate seaweed quality. Growth performance varied across sites, with DGRs ranging from 9.10 to 9.32%. Don Pale Aw yielded the highest biomass (963.00 ± 336.19 g), while Thae Chaung had the lowest (848.00 ± 152.65 g). Salinity significantly influenced growth and carrageenan yield, with higher salinities (31.25–33.15 PSU) supporting greater productivity. Growth remained stable across the temperature range (27.05–31.35°C), indicating a slight thermal tolerance over the previously established ideal range (27–29°C). The fresh-to-dry weight ratio was consistent across sites, confirming stable biomass conversion efficiency. Tissue-cultured plantlets exhibited strong adaptability to local environmental conditions, supporting the viability of large-scale K. alvarezii farming in Myanmar. Selecting sites with optimal environmental conditions (such as moderate hydrodynamics, stable salinity, high light availability, and favorable temperatures) and combining this with sustainable seaweed farming techniques has the potential to enhance local livelihoods, diversify the economy and advance Myanmar’s aquaculture sector.
References
1. Simatupang NF, Pong-Masak PR, Ratnawati P, Agusman, Paul NA, Rimmer MA. Growth and product quality of the seaweed Kappaphycus alvarezii from different farming locations in Indonesia. Aquacult Rep. 2021; 20: 100685. https://doi.org/10.1016/j.aqrep.2021.100685
2. Veeragurunathan V, Mantri VA, Vizhi JM, Eswaran K. Influence of commercial farming of Kappaphycus alvarezii (Rhodophyta) on native seaweeds of Gulf of Mannar, India: Evidence for policy and management recommendation. J Coast Conserv. 2021; 25: 51. https://doi.org/10.1007/s11852-021-00836-1
3. Hlaing WMM, Jarukamjorn K. Plantlet regeneration from callus cultures of Kappaphycus alvarezii for cultivation in coastal waters at Myeik Archipelago, Myanmar. Pak J Biol Sci. 2024; 27(9): 479–486. https://doi.org/10.3923/pjbs.2024.479.486
4. Soe-Htun U, Kyaw SPP, Wai MK, San J, Khaing SM, Aye CTPP. A review on the seaweed resources of Myanmar. J Aquac Mar Biol. 2021; 10(4): 152–166. https://doi.org/10.15406/jamb.2021.10.0031
5. Hayashi L, Reis RP, dos Santos AA, Castelar B, Robledo D, de Vega GB, Msuya FE, Eswaran K, Yasir SM, Ali MKM, Hurtado AQ. The cultivation of Kappaphycus and Eucheuma in tropical and sub-tropical waters. In Hurtado AQ, Critchley AT, Neish IC, Tropical seaweed farming trends, problems and opportunities. Developments in Applied Phycology. 2017. https://doi.org/10.1007/978-3-319-63498-2_4
6. Caceres-Farias L, Montufar-Romero M, Lodeiros C, Mercedes Espinoza-Vera M, Caceres-Farias LB, Avendano U, Ruiz-Choes W, Alfaro-Nunez A. Growth rate comparison of three Kappaphycus alvarezii colour strains cultivated using tubular netting and tie-tie methods in the waters of Ecuador. J Appl Phycol. 2025; 37: 1139–1152. https://doi.org/10.1007/s10811-025-03465-5
7. Yong YS, Yong WTL, Anton A. Analysis of formulae for determination of seaweed growth rate. J Appl Phycol. 2013; 25: 1831–1834. https://doi.org/10.1007/s10811-013-0022-7
8. Periyasamy C, Subba Rao PV, Anantharaman P. Harvest optimization to assess sustainable growth and carrageenan yield of cultivated Kappaphycus alvarezii (Doty) Doty in Indian waters. J Appl Phycol. 2019; 31: 587–597. https://doi.org/10.1007/s10811-018-1562-7
9. Hung LD, Hori K, Nang HQ, Kha T, Hoa LT. Seasonal changes in growth rate, carrageenan yield and lectin content in the red alga Kappaphycus alvarezii cultivated in Camranh Bay, Vietnam. J Appl Phycol. 2009; 21: 265–272. https://doi.org/10.1007/s10811-008-9360-2
10. Mendes M, Cotas J, Gutiérrez IB, Gonçalves AMM, Critchley AT, Hinaloc LAR, Roleda MY, Pereira L. Advanced extraction techniques and physicochemical properties of carrageenan from a novel Kappaphycus alvarezii cultivar. Mar Drugs, 2024; 22(11): 491. https://doi.org/10.3390/md22110491
11. AOAC International. Official methods of analysis of the AOAC. In Association of Official Analytical Chemists (15th ed.). Virginia: AOAC Inc; 1990.
12. Kumar YN, Poong SW, Gachon C, Brodie J, Sade A, Lim PE. Impact of elevated temperature on the physiological and biochemical responses of Kappaphycus alvarezii (Rhodophyta). PLoS ONE. 2020; 15(9): e0239097. https://doi.org/10.1371/journal.pone.0239097
13. Syamsuddin R. Seaweed Kappaphycus alvarezii ultivation for seagrass ecosystem conservation. In Gonçalves AM, Marine ecosystems – Biodiversity, ecosystem services and human impacts. 2023. http://dx.doi.org/10.5772/intechopen.106762
14. Hayashi L, Faria GSM, Nunes BG, Zitta CS, Scariot LA, Rover T, Felix MRL, Bouzon ZL. Effects of salinity on the growth rate, carrageenan yield, and cellular structure of Kappaphycus alvarezii (Rhodophyta, Gigartinales) cultured in vitro. J Appl Phycol. 2011; 23: 439–447. https://doi.org/10.1007/s10811-010-9595-6
15. Siddiqui SA, Agrawal S, Brahmbhatt H, Rathore MS. Metabolite expression changes in Kappaphycus alvarezii (a red alga) under hypo- and hyper-saline conditions. Algal Res. 2022; 63: 102650. https://doi.org/10.1016/j.algal.2022.102650
16. Hayashi L, de Paula EJ, Chow F. Growth rate and carrageenan analyses in four strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales) farmed in the subtropical waters of São Paulo State, Brazil. J Appl Phycol. 2007; 19: 393–399. https://doi.org/10.1007/s10811-006-9135-6
17. Reis RP, Loureiro RR, Mesquita FS. Does salinity affect growth and carrageenan yield of Kappaphycus alvarezii (Gigartinales/Rhodophyta)? Aquac Res. 2011; 42(8): 1231–1234. https://doi.org/10.1111/j.1365-2109.2010.02699.x
18. Rupert R, Rodrigues KF, Thien VY, Yong WTL. Carrageenan From Kappaphycus alvarezii (Rhodophyta, Solieriaceae): Metabolism, structure, production, and application. Front Plant Sci. 2022; 13 :859635. https://doi.org/10.3389/fpls.2022.859635
19. van Oort PAJ, Julianto B, Latama G, Siradjuddin I, Rukminasari N, Walyandra ZZ, Ibrahim IA, Verhagen A, van der Werf AK. Yield determinants of Kappaphycus alvarezii seaweed in South Sulawesi, Indonesia. J Appl Phycol. 2025; 37: 1153–1170. https://doi.org/10.1007/s10811-025-03446-8
20. Sarri JH, Abdulmutalib Y, Tilka MM, Terzi E, Tahiluddin A. Effects of inorganic nutrient enrichment on the carrageenan yield, growth, and ice-ice disease occurrence of red alga Kappaphycus striatus. Aquat Res. 2022; 5(2), 99–109. https://doi.org/10.3153/AR22009
21. Kasim M, Balubi AM, Astuti O, Rahman A, Patadjai RS, Muskita W, Takwir A, Ruslaini, Bahtiar, Jalil W. Comparison between the growth of Kappahycus alvarezii (Rhodophyta) seed from tissue culture and clone selection cultivated using horizontal net. Egypt J Aquat Res. 2021; 47(2): 179–184. https://doi.org/10.1016/j.ejar.2021.01.003
22. Rama R, Aslan LOM, Iba W, Nurdin AR, Armin A, Yusnaeni Y. Seaweed cultivation of micropropagated seaweed (Kappaphycus alvarezii) in bungin permai coastal waters, Tinanggea sub-district, south konawe regency, South East Sulawesi. IOP Conf Ser: Earth Environ Sci. 2018; 175: 012219. https://doi.org/10.1088/1755-1315/175/1/012219
23. Aslan LOM, Cahyani H, Hardianti H, Kurnia DP, Febriani A, Prity NA, Ariskanti, Anastasia H, Disnawati, Iba W. Field cultivation of Kappaphycus alvarezii (DOTY) doty ex silva using tissue-cultured seedlings at bungin permai costal waters, south konawe, Southeast (SE) Sulawesi: The third year of seaweed growth monitoring. IOP Conf Ser: Earth Environ Sci. 2020; 473: 012007. https://doi.org/10.1088/1755-1315/473/1/012007
24. Yong WTL, Chin JYY, Thien VY, Yasir S. Evaluation of growth rate and semi-refined carrageenan properties of tissue-cultured Kappaphycus alvarezii (Rhodophyta, Gigartinales). Phycol Res. 2014; 62(4): 316–321. https://doi.org/10.1111/pre.12067
25. Hurtado AQ, Gerung GS, Yasir S, Critchley AT. Cultivation of tropical red seaweeds in the BIMP-EAGA region. J Appl Phycol. 2014; 26: 707–718. https://doi.org/10.1007/s10811-013-0116-2
26. Periyasamy C, Anantharaman P, Balasubramanian T, Rao PVS. Seasonal variation in growth and carrageenan yield in cultivated Kappaphycus alvarezii (Doty) Doty on the coastal waters of Ramanathapuram district, Tamil Nadu. J Appl Phycol. 2014; 26: 803–810. https://doi.org/10.1007/s10811-014-0256-z
27. Ateweberhan M, Rougier A, Rakotomahazo C. Influence of environmental factors and farming technique on growth and health of farmed Kappaphycus alvarezii (cottonii) in south-west Madagascar. J Appl Phycol. 2015; 27: 923–934. https://doi.org/10.1007/s10811-014-0378-3
28. Muñoz J, Freile-Pelegrín Y, Robledo D. Mariculture of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) color strains in tropical waters of Yucatán, México. Aquaculture. 2004; 239(1–4): 161–177. https://doi.org/10.1016/j.aquaculture.2004.05.043
29. Ohno M, Largo DB, Ikumoto T. Growth rate, carrageenan yield and gel properties of cultured kappa-carrageenan producing red alga Kappaphycus alvarezzi (Doty) Doty in the subtropical waters of Shikoku, Japan. J Appl Phycol. 1994; 6: 1–5. https://doi.org/10.1007/BF02185896
30. Asni A, Najamuddin. Analysis on carrageenan content of seaweed Kappaphycus alvarezii at different water condition in Bantaeng district. IOP Conf Ser: Earth Environ Sci. 2021; 860: 012069. https://doi.org/10.1088/1755-1315/860/1/012069
31. Rui L, Jiajun L, Chaoyuan W. Effect of ammonium on growth and carrageenan content in Kappaphycus alvarezii (Gigartinales, Rhodophyta). Hydrobiologia. 1990; 204: 499–503. https://doi.org/10.1007/BF00040277
32. Li X, Li C, Liu Y, Han G, Lin C, Chen X, Mao J. Rheological and structural characterization of carrageenans during depolymerization conducted by a marine bacterium Shewanella sp. LE8. Gels. 2024; 10(8): 502. https://doi.org/10.3390/gels10080502
33. Jiang F, Liu Y, Xiao Q, Chen F, Weng H, Chen J, Zhang Y, Xiao A. Eco-friendly extraction, structure, and gel properties of ι-carrageenan extracted using Ca(OH)2. Mar Drugs. 2022; 20(7): 419. https://doi.org/10.3390/md20070419
34. Spena SR, Grizzuti N, Tammaro D. Linking processing parametrs and rheology to optimize additive manufacturing of κ-carrageenan gel systems. Gels. 2022; 8(8): 493. https://doi.org/10.3390/gels8080493
35. Necas J, Bartosikova L. Carrageenan: A review. Vet Med (Czech). 2013; 58(4): 187–205. https://doi.org/10.17221/6758-VETMED
36. Afoakwah NA, Komla MG, Ali A, Ahmed S. 24-Extraction, structural properties, and applications of carrageenan gum. Nat Gums. 2023; 2023: 647–668. https://doi.org/10.1016/B978-0-323-99468-2.00024-3
37. Avallone PR, Spena SR, Acierno S, Esposito MG, Sarrica A, Delmonte M, Pasquino R, Grizzuti N. Thermorheological behavior of κ-carrageenan hydrogels modified with xanthan gum. Fluids. 2023; 8(4),119. https://doi.org/10.3390/fluids8040119
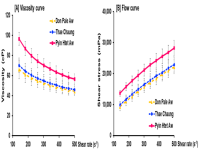
Published
Issue
Section
License
Copyright (c) 2025 Tropical Journal of Natural Product Research

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.







