Protective Effects of Peperomia pellucida Extract Against Secondhand Smoke-Induced Pulmonary Fibrosis via Antioxidant and Anti-inflammatory Pathways
Main Article Content
Abstract
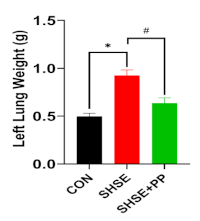
Secondhand smoke exposure (SHSE) represents a significant external risk factor, which contributes to pulmonary fibrosis onset by activating inflammatory, fibrogenic, and oxidative stress mechanisms. This study explores the efficacy of Peperomia pellucida (PP) in mitigating lung injury caused by SHSE in Wistar rats. Twenty animals were randomly grouped to one of three cohorts: control (CON), SHSE, and SHSE with PP extract (SHSE+PP). Rats were exposed to secondhand smoke for 4 consecutive weeks. The treatment group received PP extract (400 mg/kg) for 1 week before and during 4 weeks of SHSE. Lung tissues were evaluated via histopathology, immunohistochemistry, and glutathione (GSH) assays. SHSE significantly increased lung weight, histopathological scores, and profibrotic (TGF-β) and pro-inflammatory (TNF-α and IL-6) cytokines (p < 0.05). A marked reduction in GSH levels was also observed, indicating increased oxidative stress. In contrast, rats treated with PP extract showed significant improvements in all parameters, including reduced cytokine expression, improved lung architecture, and restored GSH levels (p < 0.05). These effects are likely mediated by bioactive compounds in PP, such as phenolics, phenylpropanoids, sesquiterpenes, and chlorophyll derivatives, which inhibit NF-κB and activate the Nrf2 pathway, thereby reducing inflammation, fibrosis, and oxidative damage. This is the first report demonstrating the protective effect of the extract in SHSE-induced pulmonary fibrogenesis. The results highlight the action of PP as a protective alternative medicine and support further investigation into its clinical application for preventing secondhand smoke-related chronic lung diseases.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Bellou V, Belbasis L, Evangelou E. Tobacco Smoking and Risk for Pulmonary Fibrosis. Chest. 2021;160(3):983–993.
2. Yu QY, Tang XX. Irreversibility of Pulmonary Fibrosis. Aging Dis. 2022;13(1):73.
3. Longitudinal lung function and gas transfer in individuals with idiopathic pulmonary fibrosis: a genome-wide association study. Lancet Respir Med. 2023;11(1):65–73.
4. WHO. Global Adult Tobacco Survey (GATS) Fact Sheet Indonesia 2021. 2021.
5. Estornut C, Milara J, Bayarri MA, Belhadj N, Cortijo J. Targeting Oxidative Stress as a Therapeutic Approach for Idiopathic Pulmonary Fibrosis. Front Pharmacol. 2021;12.
6. Sgalla G, Iovene B, Calvello M, Ori M, Varone F, Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res. 2018;19(1):32.
7. Soetedjo FA, Kristijanto JA, Agusaputra H, Kusumaningtyas MJ. Peperomia pellucida Extract Ameliorates Secondhand Smoke Exposure-Induced Lung Fibrogenesis via Regulation of Matrix
Metalloproteinase, Inflammatory, and Fibrotic Cytokines: A Pre-Clinical Study. Pharm Sci Asia. 2024;51(3):223–232.
8. Li Y, Zhao J, Yin Y, Li K, Zhang C, Zheng Y. The Role of IL-6 in Fibrotic Diseases: Molecular and Cellular Mechanisms. Int J Biol Sci. 2022;18(14):5405–5414.
9. Hou J, Ma T, Cao H, Chen Y, Wang C, Chen X, Xiang Z, Han X. TNF‐α‐induced NF‐κB activation promotes myofibroblast differentiation of LR‐MSCs and exacerbates bleomycin‐induced pulmonary fibrosis. J Cell Physiol. 2018;233(3):2409–2419.
10. Wang Y, Wei J, Deng H, Zheng L, Yang H, Lv X. The Role of Nrf2 in Pulmonary Fibrosis: Molecular Mechanisms and Treatment Approaches. Antioxidants. 2022;11(9):1685.
11. Gulati S, Luckhardt TR. Updated Evaluation of the Safety, Efficacy and Tolerability of Pirfenidone in the Treatment of Idiopathic Pulmonary Fibrosis. Drug Healthc Patient Saf. 2020;12:85–94.
12. Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, Johkoh T, Martinez FJ, Myers J, Protzko SL, Richeldi L, Rind D, Selman M, Theodore A, Wells AU, Hoogsteden H, Schünemann HJ; American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192(2):e3–e19.
13. Kartika IGAA, Riani C, Insanu M, Adnyana IK. Peperomia pellucida extracts stimulates bone healing in alveolar socket following tooth extraction. J Tradit Complement Med. 2022;12(3):302–307.
14. Gomes PWP, Barretto H, Reis JDE, Muribeca A, Veloso A, Albuquerque C, Teixeira A, Braamcamp W, Pamplona S, Silva C, Silva M. Chemical Composition of Leaves, Stem, and Roots of Peperomia pellucida (L.) Kunth. Molecules. 2022;27(6):1847.
15. Fakayode AE, Emma- Okon BO, Morakinyo AE, Fajobi AO, Akinyele KN, Oyedapo OO. Investigations of Ameliorative Potentials Extract of P. pellucida on Salt - Fructose Induced Dyslipidemia in Wistar Rats. Trop J Nat Prod Res. 2023;7(9):4112–4116.
16. Okoh S, Iweriebor B, Okoh O, Okoh A. Bioactive constituents, radical scavenging, and antibacterial properties of the leaves and stem essential oils from Peperomia pellucida (L.) kunth. Pharmacogn Mag. 2017;13(51):392.
17. Finato AC, Fraga-Silva TF, Prati AUC, de Souza Júnior AA, Mazzeu BF, Felippe LG, Pinto RA, Golim M de A, Arruda MSP, Furlan M, Venturini J. Crude leaf extracts of Piperaceae species downmodulate inflammatory responses by human monocytes. PLoS One. 2018;13(6):e0198682.
18. Soetedjo FA, Kristijanto JA, Khamidah N. Attenuation of Cigarette-Smoke-Induced Oxidative Stress By Oat Diet. Indones J Health Sci. 2024;8(1):91–96.
19. Kurniati I, Tjahjono K, Prasetyo A, Miranti P. I. Muntingia calabura Modulates Alveolar Matrix Metalloproteinase-9 (MMP-9) and Decreases The Alveolar Diameter in Sprague-Dawley Rats Exposed to Cigarette Smoke. Trop J Nat Prod Res. 2025;9(4):1853.
20. Ofori M, Danquah CA, Asante J, Ativui S, Doe P, Abdul-Nasir Taribabu A, Nugbemado IN, Mensah AN. Betulin and Crinum asiaticum L. bulbs extract attenuate pulmonary fibrosis by down regulating pro-fibrotic and pro-inflammatory cytokines in bleomycin-induced fibrosis mice model. Heliyon. 2023;9(6):e16914.
21. Khwaldeh A, Shraideh Z, Badran D, Shoiab A, Alsarhan AA, Al-Fawaeir S, Alzbeede A, Aljamal A. Hepatoprotective Effects of Honey Against Tobacco Smoking Toxicity in Wistar Rats. Trop J Nat Prod Res. 2024;8(5):7220–7224.
22. ThermoFisher Scientific. ELISA Sample Preparation Protocols Rat Lung Perfusion. https://www.thermofisher.com/id/en/home/references/protocols/cell-and-tissue-analysis/elisa-protocol/elisa-sample-preparation-protocols/rat-lung-perfusion.html.
23. Zhou X, Moore B. Lung Section Staining and Microscopy. Bio Protoc. 2017;7(10).
24. Klopfleisch R. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology - a systematic review. BMC Vet Res. 2013;9(1):123.
25. Lee Y-H, Seo D-S, Lee MJ, Cha H-G. Immunohistochemical characterization of oxidative stress in the lungs of rats exposed to the humidifier disinfectant polyhexamethylene guanidine hydrochloride. J Toxicol Pathol. 2019;32(4):311–317.
26. Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue – a review. Diagn Pathol. 2014;9(1):221.
27. Mishra P, Pandey C, Singh U, Gupta A, Sahu C, Keshri A. Descriptive statistics and normality tests for statistical data. Ann Card Anaesth. 2019;22(1):67.
28. Lee S, Lee DK. What is the proper way to apply the multiple comparison test? Korean J Anesthesiol. 2018;71(5):353–360.
29. Li Y, Wang L, Zhang Q, Tian L, Gan C, Liu H, Yin W, Ye T. Blueberry Juice Attenuates Pulmonary Fibrosis via Blocking the TGF-β1/Smad Signaling Pathway. Front Pharmacol. 2022;13:825915.
30. Guo J, Meng X, Zheng Y-M, Zhao S-K, Qiang C, Zhou L-B. Cigarette Smoke Mediates Nasal Epithelial Barrier Dysfunction via TNF-α. Am J Rhinol Allergy. 2023;37(6):646–655.
31. Li Y, Jiang Q, Wang L. Appetite Regulation of TLR4-Induced Inflammatory Signaling. Front Endocrinol (Lausanne). 2021;12777997.
32. Kalininskiy A, Rackow AR, Nagel D, Croft D, McGrane-Minton H, Kottmann RM. Association between weight loss and mortality in idiopathic pulmonary fibrosis. Respir Res. 2022;23(1):377.
33. Lee JK, Chung C, Kim J, Cho HS, Kim HC. Clinical impact of weight loss on mortality in patients with idiopathic pulmonary fibrosis: a retrospective cohort study. Sci Rep. 2023;13(1):5774.
34. Thakur D, Taliaferro O, Atkinson M, Stoffel R, Guleria RS, Gupta S. Inhibition of nuclear factor κB in the lungs protect bleomycin-induced lung fibrosis in mice. Mol Biol Rep. 2022;49(5):3481–3490.
35. Kim J, Cho Y, Oh G, Park H, Yang MJ, Park C, Kim Y, Choi K, Go R, Kim M. Repeated intratracheal instillation of whole‐cigarette smoke condensate to assess lung damage in a rat model. Environ Toxicol. 2024;39(4):2304–2315.
36. Fang L, Cheng Q, Zhao F, Cheng H, Luo Y, Bao X, Li Y, Liang X, Huang Y, Xu J, Han J, Tang Y, Tang S, Liu W, Luo Z, Feng D. Cigarette smoke exposure combined with lipopolysaccharides induced pulmonary fibrosis in mice. Respir Physiol Neurobiol. 2019;266:9–17.
37. Lan Y-W, Chen C-E, Huang T-T, Huang T-H, Chen C-M, Chong K-Y. Antrodia cinnamomea extract alleviates bleomycin-induced pulmonary fibrosis in mice by inhibiting the mTOR pathway. Biomed J. 2024;47(6):100720.
38. Dianat M, Radan M, Badavi M, Mard SA, Bayati V, Ahmadizadeh M. Crocin attenuates cigarette smoke-induced lung injury and cardiac dysfunction by anti-oxidative effects: the role of Nrf2 antioxidant system in preventing oxidative stress. Respir Res. 2018;19(1):58.
39. Serré J, Tanjeko AT, Mathyssen C, Vanherwegen A-S, Heigl T, Janssen R, Verbeken E, Maes K, Vanaudenaerde B, Janssens W, Gayan-Ramirez G. Enhanced lung inflammatory response in whole-body compared to nose-only cigarette smoke-exposed mice. Respir Res. 2021;22(1):86.
40. Cha S-R, Jang J, Park S-M, Ryu SM, Cho S-J, Yang S-R. Cigarette Smoke-Induced Respiratory Response: Insights into Cellular Processes and Biomarkers. Antioxidants. 2023;12(6):1210.
41. Juhl P, Bondesen S, Hawkins CL, Karsdal MA, Bay-Jensen A-C, Davies MJ, Siebuhr AS. Dermal fibroblasts have different extracellular matrix profiles induced by TGF-β, PDGF and IL-6 in a model for skin fibrosis. Sci Rep. 2020;10(1):17300.
42. Lee JH, Massagué J. TGF-β in developmental and fibrogenic EMTs. Semin Cancer Biol. 2022;86(Pt 2):136–145.
43. Ye Z, Hu Y. TGF‑β1: Gentlemanly orchestrator in idiopathic pulmonary fibrosis (Review). Int J Mol Med. 2021;48(1):132.
44. Alves NSF, Setzer WN, da Silva JKR. The chemistry and biological activities of Peperomia pellucida (Piperaceae): A critical review. J Ethnopharmacol. 2019;232:90–102.
45. Lisdiana L, Widiatningrum T, Kurniawati F. Molecular Docking Bioactive Compound of Rambutan Peel (Nephelium lappaceum L) and NF-κB in the Context of Cigarette Smoke-Induced Inflammation. Trop J Nat Prod Res. 2022;6(10):1654–1659.
46. Ho KL, Yong PH, Wang CW, Lim SH, Kuppusamy UR, Arumugam B, Ngo CT, Ng ZX. In vitro anti-inflammatory activity and molecular docking of Peperomia pellucida (L.) Kunth extract via the NF-κB and PPAR-γ signalling in human retinal pigment epithelial cells. Bioorg Chem. 2024;153:107969.
47. Nasution DLI, Tjahajawati S, Indriyanti R, Amaliya. Anti-inflammatory effectiveness of Peperomia pellucida (L.) Kunth in rats induced with periodontitis. Biochem Biophys Rep. 2024;40:101856.
48. Wang M-C. Natural plant resource flavonoids as potential therapeutic drugs for pulmonary fibrosis. Heliyon. 2023;9(8):e19308.
49. Cuijpers I, Sthijns MMJPE, van den Bogart VAR, Katsburg J, Leenders CFM, Troost FJ. Quercetin, Kaempferol and Capsaicin Counteract the TGF-β1-Induced Upregulation of αSMA and Collagen in Myoblasts. Int J Mol Sci. 2025;26(11):5151.
50. Dalle-Donne I, Garavaglia ML, Colombo G, Astori E, Lionetti MC, La Porta CAM, Santucci A, Rossi R, Giustarini D, Milzani A. Cigarette smoke and glutathione: Focus on in vitro cell models. Toxicol in Vitro. 2020;65:104818.
51. van de Wetering C, Elko E, Berg M, Schiffers CHJ, Stylianidis V, van den Berge M, Nawijn MC, Wouters EFM, Janssen-Heininger YMW, Reynaert NL. Glutathione S-transferases and their implications in the lung diseases asthma and chronic obstructive pulmonary disease: Early life susceptibility? Redox Biol. 2021;43:101995.
52. Hao W, Li M, Cai Q, Wu S, Li X, He Q, Hu Y. Roles of NRF2 in Fibrotic Diseases: From Mechanisms to Therapeutic Approaches. Front Physiol. 2022;13:889792.
53. Zhang X-L, Cao Y, Zheng B. Efficacy of N-acetylcysteine plus pirfenidone in the treatment of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. BMC Pulm Med. 2023;23(1):479.
54. Kulshrestha R, Pandey A, Jaggi A, Bansal S. Beneficial effects of N-acetylcysteine on protease-antiprotease balance in attenuating bleomycin-induced pulmonary fibrosis in rats. Iran J Basic Med Sci. 2020;23(3):396–405.