Behavioural, Biochemical, and Cerebral Histomorphological Effects of Aqueous Extract of Datura stramonium L. Leaves in Rats
Main Article Content
Abstract
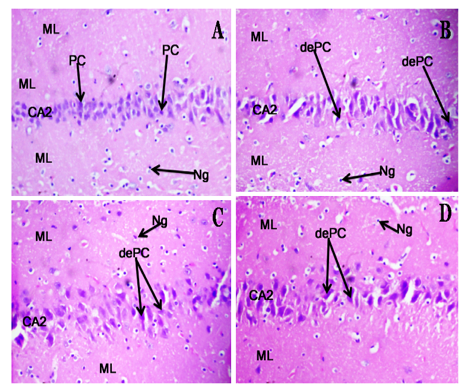
Datura stramonium L, commonly called devil's trumpet is a popular medicinal plant known for its psychoactive effect. This study aimed to investigate the behavioural, biochemical, and histomorphological changes in rats administered aqueous extract of Datura stramonium leaves. Eighty Wistar rats were divided into four groups of 20 rats per group. Group I was fed with standard rodent chow, while Groups II – IV were administered aqueous extract of Datura stramonium leaves at 50, 100, and 200 mg/kg, respectively once daily for 28 days orally. After the treatment period, the rats were subjected to behavioural tests, including conditioned place preference, open field, Y-Maze, elevated plus maze, tail suspension, and forced swim tests. After the tests, blood samples were collected for biochemical analysis, including evaluation for malondialdehyde and antioxidant enzymes (catalase and glutathione peroxidase) levels. The hippocampus and cerebral cortex were processed for histology, and brain dopamine and acetylcholine levels were determined. Results from the study showed that the sub-acute administration of the aqueous extract of Datura stramonium leaves significantly decreased feed consumption, and caused body weight loss in rats. Behavioural tests revealed that the administration of Datura stramonium leaf extract resulted in neurobehavioural symptoms like addictive tendencies, anxiety, depression, and memory impairment. In addition, the extract significantly increased brain dopamine and decreased brain acetylcholine levels. Histological examination of the brain showed degenerating pyramidal neurons in the cerebral cortex and hippocampus of rats administered Datura stramonium leaf extract. These findings suggest potential neurotoxic effect of the prolonged use of Datura stramonium leaf extract.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Yadav B, Singla A, Srivastava N, Gupta P. Pharmacognostic and Phytochemical Screening of Datura stramonium by TLC and GC-MS: A Forensic Approach. Biomed Pharmacol J. 2021; 14(4):2221-2226. https://doi.org/10.13005/bpj/2343 DOI: https://doi.org/10.13005/bpj/2320
2. Batool A, Batool Z, Qureshi R, Raja NI. Phytochemicals, Pharmacological Properties and Biotechnological Aspects of a Highly Medicinal Plant: Datura stramonium. J Plant Sci. 2020; 8(2):29-40. https://doi.org/10.11648/j.jps.20200802.11 DOI: https://doi.org/10.11648/j.jps.20200802.12
3. Choudhary M, Sharma I, Agrawal DC, Dhar MK, Kaul S. Neurotoxic Potential of Alkaloids from Thorn Apple (Datura stramonium L.): A Commonly Used Indian Folk Medicinal Herb. Med Herbs Fungi: Neurotox vs Neuroprot. 2021:391-420. https://doi.org/10.1007/978-981-16-2074-2_15 DOI: https://doi.org/10.1007/978-981-33-4141-8_16
4. Wood DM, Heyerdahl F, Yates CB, Dines AM, Giraudon I, Hovda KE, Dargan PI. The European Drug Emergencies Network (Euro-DEN). Clin Toxicol. 2014; 52(4):239-241. https://doi.org/10.3109/15563650.2014.913071 DOI: https://doi.org/10.3109/15563650.2014.898771
5. Huestis MA, Brandt SD, Rana S, Auwärter V, Baumann MH. Impact of Novel Psychoactive Substances on Clinical and Forensic Toxicology and Global Public Health. Clin Chem. 2017; 63(10):1564-1569. https://doi.org/10.1373/clinchem.2016.268409 DOI: https://doi.org/10.1373/clinchem.2017.274662
6. Das S, Kumar P, Basu SP. Phytoconstituents and Therapeutic Potentials of Datura stramonium Linn. J Drug Deliv Ther. 2012; 2(3):4-7. https://doi.org/10.22270/jddt.v2i3.168 DOI: https://doi.org/10.22270/jddt.v2i3.141
7. Devi MR, Bawari ME, Paul SB, Sharma GD. Characterization of the Toxic Effects Induced by Datura stramonium L. Leaves on Mice: A Behavioural, Biochemical and Ultrastructural Approach. Asian J Pharm Clin Res. 2012; 5(3):143-146. https://doi.org/10.22159/ajpcr.2012.v5i3.1069
8. Bouhaddouda N, Dridi A, Driouche Y, Soussa A, Otmani I, Brakni R, Kerkoub N. Chemical Composition and Biological Activities of Pituranthos Cloranthus Coss Et Dur. Essential Oil. Trop J Nat Prod Res. 2025; 9(6):2415-2420. DOI: https://doi.org/10.26538/tjnpr/v9i6.9
9. Porwal N, Gupta B, Gakkhar AK, Tiwari RC, Mittal B. An evaluation of physicochemical parameters and quantitative phytochemical analysis of Datura metel - a research article. J Ayurv Herb Integr Med. 2023; 3(2):1-13. DOI: https://doi.org/10.29121/jahim.v3.i2.2023.30
10. Jonsson M, Jestoi M, Nathanail AV, Kokkonen UM, Anttila M, Koivisto P, Karhunen P, Peltonen K. Application of OECD Guideline 423 in Assessing the Acute Oral Toxicity of Moniliformin. Food Chem Toxicol. 2013; 53:27–32. https://doi.org/10.1016/j.fct.2012.11.007 DOI: https://doi.org/10.1016/j.fct.2012.11.023
11. Uddin MK. A Review on the Adsorption of Heavy Metals by Clay Minerals, With Special Focus on the Past Decade. Chem Eng J. 2017; 308:438-462. https://doi.org/10.1016/j.cej.2016.09.029 DOI: https://doi.org/10.1016/j.cej.2016.09.029
12. Hillhouse T, Prus A. Conditioned Place Preference Test for Assessing the Rewarding Effects of Drugs of Abuse. In: Fakhoury M, eds. The Brain Reward System. Neuromethods, vol 165. Humana; 2021:10.1007/978-1-0716-1146-3_13. DOI: https://doi.org/10.1007/978-1-0716-1146-3_13
13. Amirazodi F, Mehrabi A, Amirazodi M, Parsania S, Rajizadeh MA, Esmaeilpour K. The Combination Effects of Resveratrol and Swimming HIIT Exercise on Novel Object Recognition and Open-Field Tasks in Aged Rats. Exp Aging Res. 2020; 46(4):336-358. https://doi.org/10.1080/0361073X.2020.1785702 DOI: https://doi.org/10.1080/0361073X.2020.1754015
14. Armario A. The Forced Swim Test: Historical, Conceptual and Methodological Considerations and Its Relationship with Individual Behavioural Traits. Neurosci Biobehav Rev. 2021; 128:74-86. https://doi.org/10.1016/j.neubiorev.2021.06.015 DOI: https://doi.org/10.1016/j.neubiorev.2021.06.014
15. Cryan JF, Valentino RJ, Lucki I. Assessing Substrates Underlying the Behavioural Effects of Antidepressants Using the Modified Rat Forced Swimming Test. Neurosci Biobehav Rev. 2005; 29(4-5):547-569. https://doi.org/10.1016/j.neubiorev.2004.09.004 DOI: https://doi.org/10.1016/j.neubiorev.2005.03.008
16. Ignataro P, Dicarlo M, Zerlotin R, Storlino G, Oranger A, Sanesi L, Lovero R, Buccoliero C, Mori G, Colaianni G, Colucci S. Antidepressant Effect of Intermittent Long-Term Systemic Administration of Irisin in Mice. Int J Mol Sci. 2022; 23(14):7596. https://doi.org/10.3390/ijms23147596 DOI: https://doi.org/10.3390/ijms23147596
17. Martins TM, Brown Driemeyer JP, Schmidt TP, Sobieranski AC, Dutra RC, Oliveira Weber T. A Machine Learning Approach to Immobility Detection in Mice During the Tail Suspension Test for Depressive-Type Behaviour Analysis. Res Biomed Eng. 2023; 39(1):15-26. https://doi.org/10.1007/s42600-022-00202-7 DOI: https://doi.org/10.1007/s42600-022-00246-8
18. Olofinnade AT, Onaolapo AY, Onaolapo OJ, Olowe OA. Hazelnut Modulates Neurobehaviour and Ameliorates Ageing-Induced Oxidative Stress, and Caspase-3-Mediated Apoptosis in Mice. Curr Aging Sci. 2021; 14(2):154-162. https://doi.org/10.2174/1874609814666210510104853 DOI: https://doi.org/10.2174/1874609813666201228112349
19. Saadati H, Sadegzadeh F, Sakhaie N, Panahpour H, Sagha M. Serotonin Depletion During the Postnatal Developmental Period Causes Behavioural and Cognitive Alterations and Decreases BDNF Level in the Brain of Rats. Int J Dev Neurosci. 2021; 81(2):179-190. https://doi.org/10.1016/j.ijdevneu.2021.03.004 DOI: https://doi.org/10.1002/jdn.10087
20. Yaghoubian I, Ghassemi S, Nazari M, Raei Y, Smith DL. Response of Physiological Traits, Antioxidant Enzymes and Nutrient Uptake of Soybean to Azotobacter Chroococcum and Zinc Sulfate Under Salinity. 2021; 143:42-51. https://doi.org/10.1016/j.scienta.2021.109806 DOI: https://doi.org/10.1016/j.sajb.2021.07.037
21. Wagner M, Brumelis D, Gehr R. Disinfection of Wastewater by Hydrogen Peroxide or Peracetic Acid: Development of Procedures for Measurement of Residual Disinfectant and Application to a Physicochemically Treated Municipal Effluent. Water Environ Res. 2002; 74(1):33-50. https://doi.org/10.2175/106143002X139987 DOI: https://doi.org/10.2175/106143002X139730
22. Mohamed AN, Mohammed NB, Eltom A, Abbas AO. The Relationship Between BMI and Age with Lipid Peroxidation, Antioxidant Enzyme Super Oxide Dismutase Glutathione and Serum Homocysteine Level in. Sch Int J Obstet Gynecol. 2020; 3(11):232-240. https://doi.org/10.36348/sijog.2020.v03i05.005 DOI: https://doi.org/10.36348/sijog.2020.v03i11.003
23. George-Opuda MI, Adegoke OA, Odeghe BO, Awopeju AT, Okeahialam NM. Assessment of Liver Antioxidant Profile in Plasmodium berghei Infected Mice Treated with Curative Ethanol Leaf Extract of Musa paradisiaca. Ethiop J Health Sci. 2023; 33(5):761-768. https://doi.org/10.4314/ejhs.v33i5.9 DOI: https://doi.org/10.4314/ejhs.v33i5.6
24. Huang S-J, Lin M-T, Chiang C-C, Arun Dwivedi K, Abbas A. Recent Advancements in Biological Microelectromechanical Systems (BioMEMS) and Biomimetic Coatings. Coatings. 2022; 12(12):1800. DOI: https://doi.org/10.3390/coatings12121800
25. Mthembu NA. The Role of Neuroinflammation, Serotonin Deficiency and Gene Expression in the Pathology of L-Dopa-Induced Dyskinesia with Prolonged Levodopa Treatment in a Parkinsonian Rat Model (Doctoral Dissertation). 2020. https://doi.org/10.25148/etd.FI21001898
26. Salau VF, Erukainure OL, Koorbanally NA, Islam MS. Kolaviron Modulates Dysregulated Metabolism in Oxidative Pancreatic Injury and Inhibits Intestinal Glucose Absorption with Concomitant Stimulation of Muscle Glucose Uptake. Arch Physiol Biochem. 2023; 129(1):157-167. https://doi.org/10.1080/13813455.2021.1996166 DOI: https://doi.org/10.1080/13813455.2020.1806331
27. Behl C, Moosmann B. Antioxidant Neuroprotection in Alzheimer’s Disease as Preventive and Therapeutic Approach. Free Radic Biol Med. 2002; 33(2):182-191. https://doi.org/10.1016/S0891-5849(02)00816-2 DOI: https://doi.org/10.1016/S0891-5849(02)00883-3
28. Gidado A, Zainab AA, Hadiza MU, Serah DP, Anas HY, Milala MA. Toxicity Studies of Ethanol Extract of the Leaves of Datura stramonium in Rats. Afr J Biotechnol. 2007; 6(8):1012-1015. https://doi.org/10.5897/AJB2007.000-5115
29. Zhang WN, Bast T, Xu Y, Feldon J. Temporary Inhibition of Dorsal or Ventral Hippocampus by Muscimol: Distinct Effects on Measures of Innate Anxiety on the Elevated Plus Maze, But Similar Disruption of Contextual Fear Conditioning. Behav Brain Res. 2014; 262:47-56. https://doi.org/10.1016/j.bbr.2013.09.043 DOI: https://doi.org/10.1016/j.bbr.2013.10.044
30. Bartels SJ, Pratt SI, Aschbrenner KA, Barre LK, Jue K, Wolfe RS, Xie H, McHugo G, Santos M, Williams GE, Naslund JA. Clinically Significant Improved Fitness and Weight Loss Among Overweight Persons with Serious Mental Illness. Psychiatr Serv. 2013; 64(8):729-736. https://doi.org/10.1176/appi.ps.201200280 DOI: https://doi.org/10.1176/appi.ps.003622012
31. Rana A, Samtiya M, Dhewa T, Mishra V, Aluko RE. Health Benefits of Polyphenols: A Concise Review. J Food Biochem. 2022; 46(10):e14264. https://doi.org/10.1111/jfbc.14264 DOI: https://doi.org/10.1111/jfbc.14264
32. Ramos L, Apráez JE, Cortes KS, Apráez JJ. Nutritional, Antinutritional and Phenological Characterization of Promising Forage Species for Animal Feeding in a Cold Tropical Zone. Rev Cienc Agric. 2021; 38(1):86-96. https://doi.org/10.22267/rcia.213801.151 DOI: https://doi.org/10.22267/rcia.213801.152
33. Lindenbach D, Vacca G, Ahn S, Seamans JK, Phillips AG. Optogenetic Modulation of Glutamatergic Afferents from the Ventral Subiculum to the Nucleus Accumbens: Effects on Dopamine Function, Response Vigor and Locomotor Activity. Behav Brain Res. 2022; 434:114028. https://doi.org/10.1016/j.bbr.2022.114028 DOI: https://doi.org/10.1016/j.bbr.2022.114028
34. Dela Peña I, Gevorkiana R, Shi WX. Psychostimulants Affect Dopamine Transmission Through Both Dopamine Transporter-Dependent and Independent Mechanisms. Eur J Pharmacol. 2015; 764:562-570. doi: 10.1016/j.ejphar.2015.07.044. DOI: https://doi.org/10.1016/j.ejphar.2015.07.044
35. Buck SA, Torregrossa MM, Logan RW, Freyberg Z. Roles of Dopamine and Glutamate Co‐Release in the Nucleus Accumbens in Mediating the Actions of Drugs of Abuse. FEBS J. 2021; 288(5):1462-1474. https://doi.org/10.1111/febs.15495 DOI: https://doi.org/10.1111/febs.15496
36. Jenner P, Falup-Pecurariu C, Leta V, Verin M, Auffret M, Bhidayasiri R, Weiss D, Borovečki F, Jost WH. Adopting the Rumsfeld Approach to Understanding the Action of Levodopa and Apomorphine in Parkinson’s Disease. J Neural Transm. 2023; 130(11):1337-1347. https://doi.org/10.1007/s00702-023-02618-6 DOI: https://doi.org/10.1007/s00702-023-02655-0
37. Van Roessel PJ, Grassi G, Aboujaoude EN, Menchón JM, Van Ameringen M, Rodríguez CI. Treatment-Resistant OCD: Pharmacotherapies in Adults. Compr Psychiatry. 2023; 120:152352. https://doi.org/10.1016/j.comppsych.2022.152352 DOI: https://doi.org/10.1016/j.comppsych.2022.152352
38. Nasir B, Khan AU, Baig MW, Althobaiti YS, Faheem M, Haq IU. Datura stramonium Leaf Extract Exhibits Anti-inflammatory Activity in CCL 4-Induced Hepatic Injury Model by Modulating Oxidative Stress Markers and iNOS/Nrf2 Expression. BioMed Res Int. 2022; 2022:1382878. https://doi.org/10.1155/2022/8107756 DOI: https://doi.org/10.1155/2022/1382878
39. Arias H, Targowska-Duda K. Is the Antidepressant Activity of Selective Serotonin Reuptake Inhibitors Mediated by Nicotinic Acetylcholine Receptors? Molecules. 2021; 26(8):2179. https://doi.org/10.3390/molecules26082179 DOI: https://doi.org/10.3390/molecules26082149
40. Igben VO, Iju WJ, Itivere OA, Oyem JC, Akpulu PS, Ahama EE. Datura metel stramonium Exacerbates Behavioural Deficits, Medial Prefrontal Cortex, and Hippocampal Neurotoxicity in Mice Via Redox Imbalance. Lab Anim Res. 2023; 39(1):15. https://doi.org/10.1186/s42826-023-00158-4 DOI: https://doi.org/10.1186/s42826-023-00162-7
41. Ogunsuyi OB, Ademiluyi AO, Oboh G, Agbebi OJ. Behavioural and Biochemical Indices of Neurotoxicity in Jimson Weed Administered Rats. Afr J Biomed Res. 2020; 23(1):87-96. https://doi.org/10.4314/ajbr.v23i1.13.
42. Morici JF, Weisstaub NV, Zold CL. Hippocampal-Medial Prefrontal Cortex Network Dynamics Predict Performance During Retrieval in a Context-Guided Object Memory Task. Proc Natl Acad Sci USA. 2022; 119(20):e2203024119. https://doi.org/10.1073/pnas.2203024119. DOI: https://doi.org/10.1073/pnas.2203024119
43. Huang Q, Liao C, Ge F, Ao J, Liu T. Acetylcholine Bidirectionally Regulates Learning and Memory. J Neurorestoratol. 2022; 10(2):100002. https://doi.org/10.26599/JNR.2022.1000020. DOI: https://doi.org/10.1016/j.jnrt.2022.100002
44. Kassab R. Acetylcholine-Sensitive Control of Long-Term Synaptic Potentiation in Hippocampal CA3 Neurons. Hippocampus. 2023; 33(8):948–969. https://doi.org/10.1002/hipo.23567. DOI: https://doi.org/10.1002/hipo.23533
45. Ahmad S, Khan A, Ali W, Jo MH, Park J, Ikram M, Kim MO. Fisetin Rescues the Mice Brains Against D-Galactose-Induced Oxidative Stress, Neuroinflammation and Memory Impairment. Front Pharmacol. 2021; 12:612078. https://doi.org/10.3389/fphar.2021.612078. DOI: https://doi.org/10.3389/fphar.2021.612078
46. Omidifar N, Nili-Ahmadabadi A, Nakhostin-Ansari A, Lankarani KB, Moghadami M, Mousavi SM, Hashemi SA, Gholami A, Shokripour M, Ebrahimi Z. The Modulatory Potential of Herbal Antioxidants Against Oxidative Stress and Heavy Metal Pollution: Plants Against Environmental Oxidative Stress. Environ Sci Pollut Res. 2021; 31(47):58202. https://doi.org/10.1007/s11356-021-15813-7 DOI: https://doi.org/10.1007/s11356-024-35004-z
47. Vieira JC, Bassani TB, Santiago RM, Guaita GD, Zanoveli JM, da Cunha C, Vital MA. Anxiety-Like Behaviour Induced by 6-OHDA Animal Model of Parkinson’s Disease May Be Related to a Dysregulation of Neurotransmitter Systems in Brain Areas Related to Anxiety. Behav Brain Res. 2019; 371:111981. https://doi.org/10.1016/j.bbr.2019.111981. DOI: https://doi.org/10.1016/j.bbr.2019.111981
48. DeGroot SR, Zhao-Shea R, Chung L, Klenowski PM, Sun F, Molas S, Gardner PD, Li Y, Tapper AR. Midbrain Dopamine Controls Anxiety-Like Behaviour by Engaging Unique Interpeduncular Nucleus Microcircuitry. Biol Psychiatry. 2020; 88(11):855-866. https://doi.org/10.1016/j.biopsych.2020.04.021. DOI: https://doi.org/10.1016/j.biopsych.2020.06.018
49. Tran S. Evaluation of the Catecholaminergic System in Recognition Memory (Doctoral Dissertation, University of Bristol). 2022.
50. Ranjbar-Slamloo Y, Fazlali Z. Dopamine and Noradrenaline in the Brain; Overlapping or Dissociate Functions? Front Mol Neurosci. 2020; 12:334. https://doi.org/10.3389/fnmol.2020.00334. DOI: https://doi.org/10.3389/fnmol.2019.00334
51. Morrens J, Aydin Ç, van Rensburg AJ, Rabell JE, Haesler S. Cue-Evoked Dopamine Promotes Conditioned Responding During Learning. Neuron. 2020; 106(1):142-153. https://doi.org/10.1016/j.neuron.2020.02.020. DOI: https://doi.org/10.1016/j.neuron.2020.01.012
52. Prus AJ, James JR, Rosecrans JA. Conditioned Place - Google Scholar [Internet]. [cited 2023 Oct 18]. Available from https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Prus+AJ%2C+James+JR%2C+Rosecrans+JA.+Conditioned+place+preference.+2009.&btnG= https://doi.org/10.1007/978-1-59745-503-9_7 DOI: https://doi.org/10.1007/978-1-59745-503-9_7
53. Japarin RA, Harun N, Hassan Z, Müller CP. The Dopamine D1 Receptor Antagonist SCH-23390 Blocks the Acquisition, But Not Expression of Mitragynine-Induced Conditioned Place Preference in Rats. Behav Brain Res. 2023; 453:114638. https://doi.org/10.1016/j.bbr.2023.114638 DOI: https://doi.org/10.1016/j.bbr.2023.114638
54. Ponder K. Overexpression of ΔFosB on Amphetamine Preference Behaviour: Implications for Epigenetics of Drug Seeking (Doctoral Dissertation, Saint Louis University). 2022. https://doi.org/10.13140/RG.2.2.30821.52960
55. Charalambous C, Lapka M, Havlickova T, Syslova K, Sustkova-Fiserova M. Alterations in Rat Accumbens Dopamine, Endocannabinoids and GABA Content During WIN55, 212-2 Treatment: The Role of Ghrelin. Int J Mol Sci. 2020; 22(1):210. https://doi.org/10.3390/ijms22010210 DOI: https://doi.org/10.3390/ijms22010210
56. Akuh TA, Ejembi O. Toxicological Assessment of the Ethanolic Extracts of Datura stramonium (Jimson Weed) on the Brain of Wistar Rats. Trop J Nat Prod Res. 2023; 7(5):3054-3060.
57. Bhattacharyya S, Sen P, Wallet M, Long B, Baldwin Jr AS, Tisch R. Immunoregulation of Dendritic Cells by IL-10 is Mediated Through Suppression of the PI3K/Akt Pathway and of IκB Kinase Activity. Blood. 2004; 104(4):1100-1109. https://doi.org/10.1182/blood-2003-10-3381 DOI: https://doi.org/10.1182/blood-2003-12-4302
58. Rezayof A, Ghasemzadeh Z, Sahafi OH. Addictive Drugs Modify Neurogenesis, Synaptogenesis and Synaptic Plasticity to Impair Memory Formation Through Neurotransmitter Imbalances and Signaling Dysfunction. Neurochem Int. 2023; 169:105572. https://doi.org/10.1016/j.neuint.2023.105572 DOI: https://doi.org/10.1016/j.neuint.2023.105572
59. Fasakin OW, Oboh G, Ademosun AO. The Prevalence, Mechanism of Action, and Toxicity of Nigerian Psychoactive Plants. Comp Clin Pathol. 2022; 31(5):853-873. https://doi.org/10.1007/s00580-021-03281-8 DOI: https://doi.org/10.1007/s00580-022-03374-w
60. Cao Y, Yisimayi A, Jian F, Song W, Xiao T, Wang L, Du S, Wang J, Li Q, Chen X, Yu Y. BA. 2.12. 1, BA. 4 and BA. 5 Escape Antibodies Elicited by Omicron Infection. Nature. 2022; 608(7923):593-602. https://doi.org/10.1038/s41586-022-05053-w DOI: https://doi.org/10.1038/s41586-022-04980-y
61. Skrzypczak-Wiercioch A, Sałat K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules. 2022; 27(17):5481. https://doi.org/10.3390/molecules27175481 DOI: https://doi.org/10.3390/molecules27175481
62. Bonaz B, Sinniger V, Pellissier S. Targeting the Cholinergic Anti-Inflammatory Pathway with Vagus Nerve Stimulation in Patients with Covid-19? Bioelectron Med. 2020; 6:1-7. https://doi.org/10.1186/s42234-020-00040-6 DOI: https://doi.org/10.1186/s42234-020-00051-7
63. Altindag A, Yanik M, Nebioglu M. The Comorbidity of Anxiety Disorders in Bipolar I Patients: Prevalence and Clinical Correlates. Isr J Psychiatry Relat Sci. 2006; 43(1):10. https://doi.org/10.5042/japna.2011.0499 DOI: https://doi.org/10.1080/13651500500305481