Phytochemical Profile, Antioxidant Potential, and Hemolytic Safety of Tecoma stans (L.) Kunth Leaf and Flower Extracts Using Different Solvents
Main Article Content
Abstract
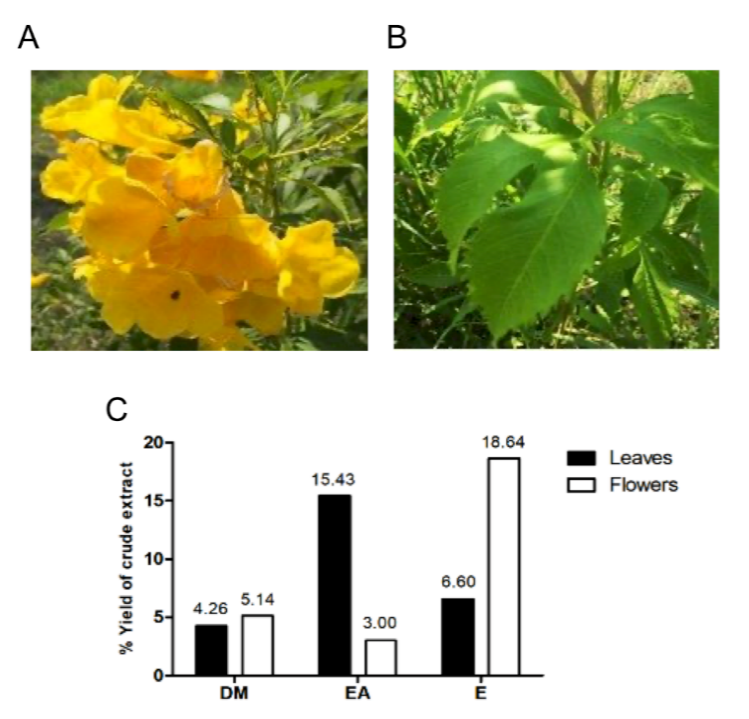
Tecoma stans, a tropical shrub, is traditionally used to treat diabetes, inflammation, infections, and digestive issues. While its antioxidant and antimicrobial effects are recognized, limited data exist on the hemolytic safety of its extracts. This study examined the phytochemical composition, antioxidant potential, and hemolytic safety of T. stans leaf and flower extracts prepared with three different solvents: dichloromethane, ethyl acetate, and 70% ethanol. Extraction yields were highest with 70% ethanol, reaching 18.64% for flowers and 6.6% for leaves extracts. Qualitative screening identified nine of ten targeted classes, including alkaloids, phenolics, flavonoids, terpenoids, and glycosides, while anthraquinones were absent in all extracts. Ethanolic extracts demonstrated the most diverse phytochemical profiles. Quantitative analysis confirmed that the ethanol leaf extract contained the highest total phenolic content (261.788 ± 6.229 mg GAE/g) and total flavonoid content (794.641 ± 259.249 mg QE/100 g). Antioxidant activity, assessed via ABTS and DPPH radical scavenging assays, showed the strongest activity in ethanol extracts. Specifically, in the ABTS assay, the ethanol leaf extract exhibited an IC₅₀ of 4.524 ± 0.122 µg/mL, surpassing the ascorbic acid standard. Similarly, the ethanol leaf extract exhibited the highest DPPH radical scavenging activity (IC₅₀ = 12.693 ± 0.207 µg/mL). Hemolysis and anti-hemolysis tests indicated that all extracts, across concentrations from 0 to 512 µg/mL, were non-hemolytic and conferred protection against red blood cell lysis. These findings suggest that T. stans, particularly its ethanol extracts, are abundant in bioactive compounds, exhibit significant antioxidant effects, and excellent hemocompatibility, supporting their potential for therapeutic or nutraceutical applications.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1 Harvey AL. Natural products in drug discovery. Drug discov today, 2008; 13(19-20): 894–901. DOI: https://doi.org/10.1016/j.drudis.2008.07.004
2 Pelton J. A Survey of the Ecology of Tecoma stans. Butl. Univ. Bot. Stud, 1964; 14: 11.
3 Al-Azzawi A, Al-Khateeb E, Al-Sameraei K, Al-Juboori A. Antibacterial activity and the histopathological study of crude extracts and isolated tecomine from Tecoma stans Bignoniaceae in Iraq. J. Pharmacogn. Res, 2012; 4: 37–43. DOI: https://doi.org/10.4103/0974-8490.91033
4 Aguilar LC, Macias S, Chagoya A, Cardenas A, Diaz P, Cantu JM. Antidiabetic activity of Tecoma stans in rats. Fitoterapia, 1993; 64: 304–305.
5 Salem MZM, Gohar YM, Camacho LM, El-Shanhorey NA, Salem AZM. Antioxidant and antibacterial activities of leaves and branches extracts of Tecoma stans (L.) Juss. ex Kunth against nine species of pathogenic bacteria. Afr. J. Microbiol. Res, 2013; 7: 418–426. DOI: https://doi.org/10.5897/AJMR12.2274
6 Gupta A, Behl T, Sehgal A, Singh S, Sharma N, Yadav S, Anwer K, Cruz CV, Chigurupati S, Farasani A, Bhatia S. Elucidating the Neuroprotective Effect of Tecoma stans Leaf Extract in STZ-Induced Diabetic Neuropathy. Evid Based Complement Alternat Med, 2022; 3833392. DOI: https://doi.org/10.1155/2022/3833392
7 Choudhury S, Dipjyoti C. Phenolic and Flavonoid Content and Antioxidant Activity, of Three Different Extracts of Tecoma Stans (L.) Kunth and Zingiber Officinales Roscoe. Asian Plant R J, 2023; 11(6):119-125. DOI: https://doi.org/10.9734/aprj/2023/v11i6236
8 Krobthong S, Yingchutrakul Y, Sittisaree W, Tulyananda T, Samutrtai P, Choowongkomon K, Lao-On U. Evaluation of potential anti-metastatic and antioxidative abilities of natural peptides derived from Tecoma stans (L.) Juss. ex Kunth in A549 cells. PeerJ, 2022; 10: e13693. DOI: https://doi.org/10.7717/peerj.13693
9 Al-Azzawi A, Al Dibsawi A, Talath S, Wali AF, Sarheed O. Method Development: The Antioxidant and Antifungal Activity of the Isolated Component from the Ethanolic Extract of Tecoma stans Leaves Using Flash Chromatography. Separations, 2022; 9(10): 317. DOI: https://doi.org/10.3390/separations9100317
10 Das C, Dash S, Sahoo DC, Mohanty A. Evaluation of methanolic bark extract of Tecoma stans for wound healing in albino rats. Inter Jof Pharm and Tech. 2010; 2(3):735–742
11 Durgadevi T, Devika K, Anburaj G, Valliammai CT. To determine the antioxidant mechanism of action, biochemical compounds in Tecoma stans (L. Juss. ex Kunth) were analyzed using GC-MS, HPLC, UV-VIS, and FTIR techniques. Eco. Env. & Cons. 2024; 30: S460-S466. DOI: https://doi.org/10.53550/EEC.2024.v30i06s.067
12 Singh S, Miller CT, Singh P, Sharma R, Rana N, Dhakad AK, Dubey RK. A comprehensive review on ecology, life cycle and use of Tecoma stans (bignoneaceae). Bot stud, 2024; 65(1):6. DOI: https://doi.org/10.1186/s40529-024-00412-4
13 So-In C, Sunthamala N. Treatment efficacy of Thunbergia laurifolia, Curcuma longa, Garcinia mangostana, and Andrographis paniculata extracts in Staphylococcus aureus-induced rabbit dermatitis model. Vet World. 2022; 15(1):188-197. DOI: https://doi.org/10.14202/vetworld.2022.188-197
14 Sunthamala N, Sankla N, Seedakaew P, Senasuk A, Matturee J, Suksilp W, Sunthamala P, Yonyubon S, Phetsom J, Maneerat S, Thiwthong R, Khankhum S. Achyranthes aspera Linn. crude extracts enhance phagocytic activity and attenuate oxidative stress-mediated-TNF-α mRNA expression in primary human monocyte-derived macrophage. Trop J Nat Prod Res. 2021; 5(9):1569-1577 DOI: https://doi.org/10.26538/tjnpr/v5i9.8
15 Okolie NP, Falodun A, Oluseyi D. Evaluation of the antioxidant activity of root extract of pepper fruit (Dennetia tripetala), and its potential for the inhibition of Lipid peroxidation. Afri J Trad Complem and Altern Med. 2014; 11(3):221-227. DOI: https://doi.org/10.4314/ajtcam.v11i3.31
16 Ayoola G, Coker H, Adesegun S, Bello AA, Obaweya K, Ezennia E, Atangbayila T. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharm Res. 2008; 7(3):1019-1024. DOI: https://doi.org/10.4314/tjpr.v7i3.14686
17 Sankla N, Sunthamala N. Phytochemical screening, Antioxidant activity and ex vivo immunological-associated properties of a halophyte extract, Maytenus mekongensis In Sched. Trop J Nat Prod Res. 2021; 5(11):1949-1957. DOI: https://doi.org/10.26538/tjnpr/v5i11.10
18 So-In C, Sunthamala N. The effects of mulberry (Morus alba Linn.) leaf supplementation on growth performance, blood parameter, and antioxidant status of broiler chickens under high stocking density. Vet World. 2022; 15(11):2715–2724. DOI: https://doi.org/10.14202/vetworld.2022.2715-2724
19 Odion EE, Falodun A and Adelusi SA. Total flavonoid, Total Phenolic and antioxidant potential of root bark extract and fractions of from Cola rostrata (Sterculiaceae) K. Schum. University of Benin 2013; J. Sci. Tech 1 (2): 38 - 42.
20 Iheanacho CM, Akubuiro PC, Oseghale IO, Imieje VO, Erharuyi O, Ogbeide KO, Jideonwo AN, Falodun A. Evaluation of the Antioxidant Activity of the Stem Bark Extracts of Anacardium occidentale (Linn) Anacardiaceae. Trop J Nat Prod Res. 2023; 2(2):65-69. DOI: https://doi.org/10.26538/tjpps/v2i2.4
21 So-In C, Khankhum S, Khaowong I, Pangchai T, Sunthamala N. Efficacy of Garcinia mangostana Linn. and Achyranthes aspera Linn. combined extracts in the prevention of endometritis in cattle. Trop Anim Sci J. 2024; 47(3):291-299. DOI: https://doi.org/10.5398/tasj.2024.47.3.291
22 Sankla N, Loutchanwoot P, Khankhum S, Khammuang S, Sarnthima R, Sunthamala N. In vitro antioxidant and immunological-associated activities of ethanol extracts of Azima sarmentosa (Blume) Benth. & Hook. F. Trop J Nat Prod Res. 2022; 6(12):2007–2013.
23 Egharevba E, Chukwuemeke-Nwani P, Eboh U, Okoye E, Bolanle IO, Oseghale IO, Imieje VO, Erharuyi O, Falodun A. Antioxidant and Hypoglycaemic Potentials of the Leaf Extracts of Stachytarphyta jamaicensis (Verbenaceae). Trop J Nat Prod Res. 2019; 3(5):170-174. DOI: https://doi.org/10.26538/tjnpr/v3i5.4
24 Sunthamala N, Suebsamran C, Khruaphet N, Sankla N, Janpirom J, Thiwthong R, Chuncher S, Khankhum S. Sanguinarine and chelidonine synergistically induce endosomal toll-like receptor and M1-associated mediators expression. J Pure Appl Microbiol. 2020; 14(4):2351-2361. DOI: https://doi.org/10.22207/JPAM.14.4.13
25 Karim A, Islam A, Islam M, Rahman S, Sultana S, Biswas S, Jakir Hosen M, Mazumder K, Rahman M, Hasan N. Evaluation of antioxidant, anti-hemolytic, cytotoxic effects and anti-bacterial activity of selected mangrove plants (Bruguiera gymnorrhiza and Heritiera littoralis) in Bangladesh. Clin Phytosci. 2020; 6(8):1-12. DOI: https://doi.org/10.1186/s40816-020-0152-9
26 So-In C, Pangchai T, Sunthamala P, Maneerat S, Khankhum S, Sunthamala N. Phytochemical composition, Antioxidant Activity, and Cytotoxicity of Asystasia gangetica (Linn) T. Anderson Extracts . Trop J Nat Prod Res, 2025; 9(5): 2227-2233. DOI: https://doi.org/10.26538/tjnpr/v9i5.50
27 Ngoupayo J, Talla C, Dongmo YKM, Bijou-Lafortune NK, Boyom FF, Mpondo EM. Unravelling the Antioxidant and Antimicrobial Effect of Tecoma Stans Extracts. Sch Acad J Pharm, 2024; 13(7): 317-325. DOI: https://doi.org/10.36347/sajp.2024.v13i07.004
28 Narayanan M, Gothandapani A, Venugopalan R, Rethinam M, Pitchai S, Alahmadi TA, Almoallim HS, Kandasamy S, Brindhadevi K. Antioxidant and anticancer potential of ethyl acetate extract of bark and flower of Tecoma stans (Linn) and In Silico studies on phytoligands against Bcl 2 and VEGFR2 factors. Environ Res, 2023; 231:116112. DOI: https://doi.org/10.1016/j.envres.2023.116112
29 Bors W, Michel C, Stettmaier K. Structure-activity relationships governing antioxidant capacities of plant polyphenols. Methods enzymol, 2001; 335:166–180. DOI: https://doi.org/10.1016/S0076-6879(01)35241-2
30 Wichayapreechar P, Prasansuklab A, Charoongchit P, Charoenjittichai R. The Potential of Tecoma stans (Linn.) Flower Extract as a Natural Antioxidant and Anti-Aging Agent for Skin Care Products. Cosmetics, 2024; 11:214. DOI: https://doi.org/10.3390/cosmetics11060214
31 Gupta A, Behl T. Proposed mechanism of Tecoma stans in diabetes-associated complications, Nat Prod J, 2021; 11(2):127139. DOI: https://doi.org/10.2174/2210315510666191224114311