Anticancer Activity of Combined Extracts of Sappan Wood (Caesalpinia sappan L.) and Coffee Stem Parasite (Loranthus ferrugineus Roxb) Against MCF7 Breast Cancer Cells Through In Silico and In Vitro Approaches
Main Article Content
Abstract
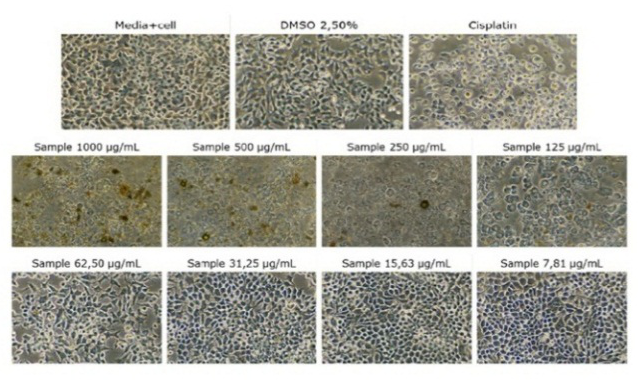
Breast cancer remains the most prevalent malignancy among women worldwide. Adverse side effects associated with conventional cancer therapies have driven the search for alternative chemopreventive agents from natural sources. Caesalpinia sappan L. and Loranthus ferrugineus Roxb. have long been used in traditional medicine and possess anticancer properties. This study aimed to evaluate the anticancer activity of single and combined extracts of C. sappan L. and L. ferrugineus Roxb. against Michigan Cancer Foundation-7 (MCF-7) breast cancer cells and elucidate their molecular mechanisms via in silico docking simulations. In silico screening focused on brazilin and quercetin, the principal phytoconstituents of C. sappan L. and L. ferrugineus Roxb., respectively, compared with a standard drug targeting cytochrome P450 3A4 (CYP3A4, PDB ID: 1TQN) using PyRx AutoDock Vina. The resazurin reduction assay was used to determine in vitro cytotoxicity against MCF-7 cells using acetone extracts of L. ferrugineus Roxb., C. sappan L., and their combinations at ratios of 1:1, 2:1, and 1:2. In silico results indicated that brazilin and quercetin complied with Lipinski’s Rule of Five, with binding affinities of −8.2 kcal/mol and −8.9 kcal/mol, respectively, whereas their combination violated the rule. While both compounds individually exhibited promising anticancer potential, their combination showed a competitive antagonistic effect. In vitro findings showed that C. sappan L. extract exerted moderate cytotoxicity (IC50 = 56.21 µg/mL), whereas L. ferrugineus Roxb. extract had an IC50 of 112.9 µg/mL. All extract combinations were inactive, indicating antagonistic interactions between the secondary metabolites of both plants.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263. Doi:10.3322/caac.21834 DOI: https://doi.org/10.3322/caac.21834
2. Saini A, Kumar M, Bhatt S, Saini V, Malik A. Cancer Causes and Treatments. Int J Pharm Sci Res. 2020;11(7):3109. Doi:10.13040/IJPSR.0975-8232.11(7).3109-22 DOI: https://doi.org/10.13040/IJPSR.0975-8232.11(7).3109-22
3. Tubin S, Vozenin MC, Prezado Y, Durante M, Prise KM, Lara PC, Greco C, Massaccesi M, Guha C, Wu X, Mohiuddin MM, Vestergaard A, Bassler N, Gupta S, Stock M, Timmerman R. Novel unconventional radiotherapy techniques: Current status and future perspectives – Report from the 2nd international radiation oncology online seminar. Clin Transl Radiat Oncol. 2023;40:100605. Doi:10.1016/j.ctro.2023.100605 DOI: https://doi.org/10.1016/j.ctro.2023.100605
4. Juwitaningsih T, Syahputra N, Eddiyanto E, Windayani N, Rukayadi Y. Antibacterial and Anticancer Activities of Acetone Extract Caesalpinia sappan L. al-Kimiya. 2023;9(2):82-88. Doi:10.15575/ak.v9i2.20966 DOI: https://doi.org/10.15575/ak.v9i2.20966
5. Suyatmi S, Mudigdo A, Purwanto B, Indarto D, Hakim F, Krisnawati D. Brazilin Isolated from Caesalpina Sappan Wood Induces Intrinsic Apoptosis on A549 Cancer Cell Line by Increasing p53, caspase-9, and caspase-3. Asian Pac J Cancer Prev. 2022;23(4):1337-1343. Doi:10.31557/APJCP.2022.23.4.1337
6. Astirin OP, Artanti AN, Herawati E, Putri FA, Muhyidin MF, Prihapsara F. Cytotoxic effects of fractions, isolates, and nanoparticles of soursop leaf (Annona muricata L.) and sappan wood (Caesalpinia sappan L.) against human cervical cancer HeLa cells. Indian J Nat Prod Resour. Published online 2023. Doi:10.56042/ijnpr.v14i4.5779 DOI: https://doi.org/10.56042/ijnpr.v14i4.5779
7. Yang X, Liang Y, Zhao L, Chen L, Yang Y, Wang J, Yan L, Zhang S, Liu X, Zhang H. Brazilin Inhibits the Invasion and Metastasis of Breast Cancer. Biol Pharm Bull. 2023;46(6):b22-00637. Doi:10.1248/bpb.b22-00637 DOI: https://doi.org/10.1248/bpb.b22-00637
8. Jeong M, Chun J, Park SM, Yeo H, Na SW, Ha IJ, Kim B, Jeong MK. An Investigation of the Anticancer Mechanism of Caesalpinia sappan L. Extract Against Colorectal Cancer by Integrating a Network Pharmacological Analysis and Experimental Validation. Plants. 2025;14(2):263. Doi:10.3390/plants14020263 DOI: https://doi.org/10.3390/plants14020263
9. Juwitaningsih T, Roza D, Silaban S, Hermawati E, Windayani N. Phytochemical screening, antibacterial, antioxidant, and anticancer activity of Coffee parasite acetone extract (Loranthus ferrugineus Roxb). Pharmacia. 2022;69(4):1041-1046. Doi:10.3897/pharmacia.69.e91427 DOI: https://doi.org/10.3897/pharmacia.69.e91427
10. Nigam YP. The Bornean Mistletoes as Versatile Parasites: A Systematic Review. Sys Rev Pharm. 2022; 13(1): 42-47. Doi:10.31858/0975-8453.13.1.42-47
11. Niazvand F, Orazizadeh M, Khorsandi L, Abbaspour M, Mansouri E, Khodadadi A. Effects of Quercetin-Loaded Nanoparticles on MCF-7 Human Breast Cancer Cells. Medicina (B Aires). 2019;55(4):114. Doi:10.3390/medicina55040114 DOI: https://doi.org/10.3390/medicina55040114
12. Suyatmi S, Mudigdo A, Purwanto B, Indarto D, Hakim F, Krisnawati D. Brazilin Isolated from Caesalpina Sappan Wood Induces Intrinsic Apoptosis on A549 Cancer Cell Line by Increasing p53, caspase-9, and caspase-3. Asian Pac J Cancer Prev. 2022;23(4):1337-1343. Doi:10.31557/APJCP.2022.23.4.1337 DOI: https://doi.org/10.31557/APJCP.2022.23.4.1337
13. Dewi Harnis AP, A.H.M. Hasan N, Janah YK, Tsamara CA, Fatchiyah F. Virtual Inhibition Analysis of Bioactive Compound Brazilin (Caesalpinia sappan L.) Toward Progesterone Receptor or Lonaprisan in Breast Cancer Proliferation. Biotrop: j. tropical biol. 2020;8(2):62-70. doi:10.21776/ub.biotropika.2020.008.02.01 DOI: https://doi.org/10.21776/ub.biotropika.2020.008.02.01
14. Tirtanirmala P, Novarina A, Utomo RY, Sugiyanto RN, Jenie RI, Meiyanto E. Cytotoxic and Apoptotic-inducing Effect of Fraction Containing Brazilein from Caesalpinia sappan L. and Cisplatin on T47D Cell Lines. Indones. J. Cancer Chemoprevention. 2017;6(3):89-96. Doi:10.14499/indonesianjcanchemoprev6iss3pp89-96 DOI: https://doi.org/10.14499/indonesianjcanchemoprev6iss3pp89-96
15. Thomaz DV, Rodrigues ESB, Machado FB, Macedo IYL. Investigation of Cyclobenzaprine Interactions with P450 Cytochromes CYP1A2 and CYP3A4 through Molecular Docking Tools. Path Sci. 2019;5(2):4001-4006. Doi:10.22178/pos.43-1 DOI: https://doi.org/10.22178/pos.43-1
16. Gor PP, Su HI, Gray RJ, Gimotty PA, Horn M, Aplenc R, Vaughan WP, Tallman MS, Rebbeck TR, DeMichele A. Cyclophosphamide- metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. Doi:10.1186/bcr2570 DOI: https://doi.org/10.1186/bcr2570
17. El-Serafi I, Steele S. Cyclophosphamide Pharmacogenomic Variation in Cancer Treatment and Its Effect on Bioactivation and Pharmacokinetics. Adv Pharmacol Pharm Sci. 2024;2024:4862706. Doi:10.1155/2024/4862706 DOI: https://doi.org/10.1155/2024/4862706
18. Muthi I, Riris Istighfari J, Rohmad Yudi U, Nur Dina A, Gagas Pradani Nur I, Masashi K, Edy M. Genistein enhances cytotoxic and antimigratory activities of doxorubicin on 4T1 breast cancer cells through cell cycle arrest and ROS generation. J Appl Pharm Sci. 2020;10(10):095-104. Doi:10.7324/JAPS.2020.1010011 DOI: https://doi.org/10.7324/JAPS.2020.1010011
19. Mahmoud AM, El-Shemy HA. Efficacy Assessment for Several Natural Products with Potential Cytotoxic Activity Against Breast and Cervix Cancers. J. Arid. Land. 2012;22(1):107-110.
20. Zubair MS, Anam S, Maulana S, Arba M. In Vitro and In Silico Studies of Quercetin and Daidzin as Selective Anticancer Agents. Indones. J. Chem. 2021;21(2):310-317. Doi:10.22146/ijc.53552 DOI: https://doi.org/10.22146/ijc.53552
21. Tang SM, Deng XT, Zhou J, Li QP, Ge XX, Miao L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed Pharmacother. 2020;121:109604. Doi:10.1016/j.biopha.2019.109604 DOI: https://doi.org/10.1016/j.biopha.2019.109604
22. Biswas P, Dey D, Biswas PK, Rahaman TI, Saha S, Parvez A, Khan DA, Lily NJ, Saha K, Sohel M, Hasan MM, Al Azad S, Bibi S, Hasan MN, Rahmatullah M, Chun J, Rahman MA, Kim B. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int J Mol Sci. 2022;23(19). Doi:10.3390/ijms231911746 DOI: https://doi.org/10.3390/ijms231911746
23. Forli S, Huey R, Pique ME, Sanner MF, Goodsell DS, Olson AJ. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat Protoc. 2016;11(5):905-919. Doi:10.1038/nprot.2016.051 DOI: https://doi.org/10.1038/nprot.2016.051
24. Lavogina D, Lust H, Tahk MJ, Laasfeld T, Vellama H, Nasirova N, Vardja M, Eskla KL, Salumets A, Rinken A, Jaal J. Revisiting the Resazurin-Based Sensing of Cellular Viability: Widening the Application Horizon. Biosensors (Basel). 2022;12(4):196. doi:10.3390/bios12040196
25. Susianti S, Lesmana R, Salam S, Julaeha E, Pratiwi YS, Sylviana N, Goenawan H, Kurniawan A, Supratman U. The Effect of Nutmeg Seed (M. fragrans) Extracts Induces Apoptosis in Melanoma Maligna Cell’s (B16-F10). Indones. Biomed. J.. 2021;13(1):68-74. Doi:10.18585/inabj.v13i1.1424 DOI: https://doi.org/10.18585/inabj.v13i1.1424
26. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings 1PII of original article: S0169-409X(96)00423-1. Adv Drug Deliv Rev. 2001;46(1-3):3-26. Doi:10.1016/S0169-409X(00)00129-0 DOI: https://doi.org/10.1016/S0169-409X(00)00129-0
27. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7(1):42717. Doi:10.1038/srep42717 DOI: https://doi.org/10.1038/srep42717
28. Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455-461. Doi:10.1002/jcc.21334
29. Roza D, Fadhilah G, Indriani E, Wiliranti YA, Juwitaningsih T. Evaluation of Anticancer and Antioxidant Activities (In Vitro Studies) of Coffee Stem Parasite Extract [Scurrula ferruginea (Roxb. ex Jack) Danser] and In Silico Studies of its Isolate. Turk J Pharm Sci. 2024;21(5):463-473. Doi:10.4274/tjps.galenos.2023.26243 DOI: https://doi.org/10.4274/tjps.galenos.2023.26243
30. Karami TK, Hailu S, Feng S, Graham R, Gukasyan HJ. Eyes on Lipinski’s Rule of Five: A New “Rule of Thumb” for Physicochemical Design Space of Ophthalmic Drugs. J Ocul Pharmacol Ther. 2022;38(1):43-55. Doi:10.1089/jop.2021.0069 DOI: https://doi.org/10.1089/jop.2021.0069
31. Yano JK, Wester MR, Schoch GA, Griffin KJ, Stout CD, Johnson EF. The Structure of Human Microsomal Cytochrome P450 3A4 Determined by X-ray Crystallography to 2.05-Å Resolution. J. biol. chem. 2004;279(37):38091-38094. Doi:10.1074/jbc.C400293200 DOI: https://doi.org/10.1074/jbc.C400293200
32. Ekroos M, Sjögren T. Structural basis for ligand promiscuity in cytochrome P450 3A4. Proc Natl Acad Sci U S A. 2006;103(37):13682-13687. Doi:10.1073/pnas.0603236103 DOI: https://doi.org/10.1073/pnas.0603236103
33. Tang H, Kuang Y, Wu W, Peng B, Fu Q. Quercetin inhibits the metabolism of arachidonic acid by inhibiting the activity of CYP3A4, thereby inhibiting the progression of breast cancer. Mol Med. 2023;29(1):127. Doi:10.1186/s10020-023-00720-8 DOI: https://doi.org/10.1186/s10020-023-00720-8
34. Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455-461. Doi:10.1002/jcc.21334 DOI: https://doi.org/10.1002/jcc.21334
35. Kiani YS, Ranaghan KE, Jabeen I, Mulholland AJ. Molecular Dynamics Simulation Framework to Probe the Binding Hypothesis of CYP3A4 Inhibitors. Int J Mol Sci. 2019;20(18):4468. Doi:10.3390/ijms20184468 DOI: https://doi.org/10.3390/ijms20184468
36. Ridhwan MJM, Bakar SIA, Latip NA, Ghani NA, Ismail NH. A Comprehensive Analysis of Human CYP3A4 Crystal Structures as a Potential Tool for Molecular Docking-Based Site of Metabolism and Enzyme Inhibition Studies. J Comput Biophys Chem. 2022;21(03):259-285. Doi:10.1142/S2737416522300012 DOI: https://doi.org/10.1142/S2737416522300012
37. Wang J, Nithianantham S, Chai SC, Jung YH, Yang L, Ong HW, Li Y, Zhang Y, Miller DJ, Chen T. Decoding the selective chemical modulation of CYP3A4. Nat Commun. 2025;16(1):3423. Doi:10.1038/s41467-025-58749-8 DOI: https://doi.org/10.1038/s41467-025-58749-8
38. Dhiman N, Awasthi R, Sharma B, Kharkwal H, Kulkarni GT. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front Chem. 2021;9. Doi:10.3389/fchem.2021.580118 DOI: https://doi.org/10.3389/fchem.2021.580118
39. Pinheiro RGR, Pinheiro M, Neves AR. Nanotechnology Innovations to Enhance the Therapeutic Efficacy of Quercetin. Nanomaterials. 2021;11(10):2658. Doi:10.3390/nano11102658 DOI: https://doi.org/10.3390/nano11102658
40. Lavogina D, Lust H, Tahk MJ, Laasfeld T, Vellama H, Nasirova N, Vardja M, Eskla KL, Salumets A, Rinken A, Jaal J. Revisiting the Resazurin-Based Sensing of Cellular Viability: Widening the Application Horizon. Biosensors (Basel). 2022;12(4). Doi:10.3390/bios12040196 DOI: https://doi.org/10.3390/bios12040196
41. Pezzuto JM, Song LL, Lee SK, Shamon LA, Mata-Greenwood E, Jang M, Jeong HJ, Pisha E, Mehta RG, Kinghorn AD. Bioassay Methods Useful for Activity-Guided Isolation of Natural Product Cancer Chemopreventive Agents. In: Chemistry, Biological and Pharmacological Properties of Medicinal Plants from the Americas. Routledge; 2018:81-110. Doi:10.1201/9781315139272-5 DOI: https://doi.org/10.1201/9781315139272-5
42. Juwitaningsih T, Jahro IS, Sari SA. Evaluation of North Sumatera Cardamom seed (Amomum compactum) Extract as Antibacterial and Anticancer. J Phys Conf Ser. 2020;1485(1):012019. doi:10.1088/1742-6596/1485/1/012019 DOI: https://doi.org/10.1088/1742-6596/1485/1/012019
43. Kumal K, Pant DR, Aryal B, Tripathi GR, Joshi GP. Phytochemical and Antioxidant Properties Of Traditionally Used Mistletoes In Nepal. Sci World. 2021;14(14):83-89. Doi:10.3126/sw.v14i14.34999 DOI: https://doi.org/10.3126/sw.v14i14.34999
44. Chatterjee B, Ghosh K, Swain A, Nalla KK, Ravula H, Pan A, Kanade SR. The phytochemical brazilin suppress DNMT1 expression by recruiting p53 to its promoter resulting in the epigenetic restoration of p21 in MCF7cells. Phytomedicine. 2022;95:153885. Doi:10.1016/j.phymed.2021.153885 DOI: https://doi.org/10.1016/j.phymed.2021.153885
45. Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as Anticancer Agents. Nutrients. 2020;12(2):457. Doi:10.3390/nu12020457 DOI: https://doi.org/10.3390/nu12020457
46. Engel N, Oppermann C, Falodun A, Kragl U. Proliferative effects of five traditional Nigerian medicinal plant extracts on human breast and bone cancer cell lines. J Ethnopharmacol. 2011;137(2):1003-1010. Doi:10.1016/j.jep.2011.07.023 DOI: https://doi.org/10.1016/j.jep.2011.07.023
47. Tang J, Wennerberg K, Aittokallio T. What is synergy? The Saariselkä agreement revisited. Front Pharmacol. 2015;6(181). Doi:10.3389/fphar.2015.00181 DOI: https://doi.org/10.3389/fphar.2015.00181
48. Canga I, Vita P, Oliveira AI, Castro MÁ, Pinho C. In Vitro Cytotoxic Activity of African Plants: A Review. Molecules. 2022;27(15). Doi:10.3390/molecules27154989 DOI: https://doi.org/10.3390/molecules27154989