Decaffeinated Green Tea and Green Coffee Extract Attenuate Cardiac Perivascular Fibrosis in a Metabolic Syndrome Model by Decreasing Fibroblast Growth Factor 23 and Runt-related transcription factor 2 Expression
Main Article Content
Abstract
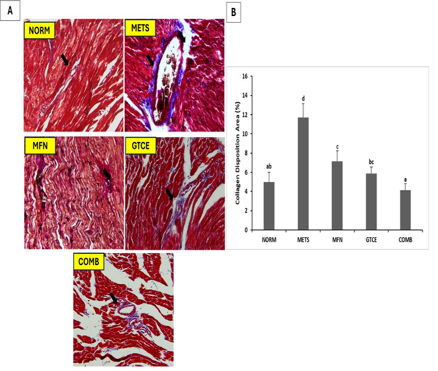
Metabolic syndrome includes hypertension, obesity, and insulin resistance, which increase the risk of cardiovascular disease (CVD) by up to 50%. This condition activates genes, such as FGF23, GALNT3, and RUNX2, causing heart fibrosis. This study aimed to determine the effect of tea and coffee extract therapy on perivascular fibrosis in the heart of a mouse model of metabolic syndrome and its impact on the expression of fibrosis-related genes such as FGF23, GALNT3, and RUNX2. This study used 25 male Sprague Dawley rats, divided into five groups (n=5): negative control (NORM), positive control (METS), metformin therapy (MFN), green tea and green coffee extract therapy (GTCE), and a combination of both (COMB). The METS samples were fed a high-fat and high-sucrose diet for 18 weeks, followed by a low-dose Streptozotocin injection (30 mf/kgBW) for 11 weeks. The METS model was then administered treatment for 9 weeks. After treatment, the rats were dissected, and the heart organs were analyzed with Masson Trichrome, and FGF23, GALNT3, and RUNX2 mRNA expression was measured by RT-PCR. The results showed that green tea and coffee extracts, alone or in combination with metformin, showed anti-fibrotic effects by reducing collagen deposition (5.87% ± 0.66 and 4.14% ± 0.66) and lowering FGF23 (0.543 ± 0.112 and 0.676 ± 0.159) and RUNX2 (2.716 ± 0.482 and 7.325 ± 0.899). These results suggest that combination extracts exhibit anti-fibrotic effects by reducing collagen deposition in perivascular area. They also suppress pro-fibrotic genes, such as FGF23 and RUNX2, which are involved in cardiac fibrosis.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. National Cholesterol Education Program (NCEP). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106(25):3143–3143. DOI: https://doi.org/10.1161/circ.106.25.3143
2. Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11(8):215–225. DOI: https://doi.org/10.1177/1753944717711379
3. Alipour P, Azizi Z, Raparelli V, Norris CM, Kautzky-Willer A, Kublickiene K, Herrero MT, Emam KE, Vollenweider P, Preisig M, Clair C, Pilote L. Role of sex and gender-related variables in development of metabolic syndrome: A prospective cohort study. Eur J Intern Med. 2024;121:63–75. DOI: https://doi.org/10.1016/j.ejim.2023.10.006
4. Ou YJ, Lee JI, Huang SP, Chen SC, Geng JH, Su CH. Association between Menopause, Postmenopausal Hormone Therapy and Metabolic Syndrome. J Clin Med. 2023;12(13):4435. DOI: https://doi.org/10.3390/jcm12134435
5. Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, Nkeck JR, Nyaga UF, Ngouo AT, Tounouga DN, Tianyi FL, Foka AJ, Ndoadoumgue AL, Bigna JJ. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res Clin Pract. 2022;188:109924. DOI: https://doi.org/10.1016/j.diabres.2022.109924
6. Herningtyas EH, Ng TS. Prevalence and distribution of metabolic syndrome and its components among provinces and ethnic groups in Indonesia. BMC Public Health. 2019;19(1):377. DOI: https://doi.org/10.1186/s12889-019-6711-7
7. Qiao Q, Gao W, Zhang L, Nyamdorj R, Tuomilehto J. Metabolic syndrome and cardiovascular disease. Ann Clin Biochem Int J Lab Med. 2007;44(3):232–263. DOI: https://doi.org/10.1258/000456307780480963
8. Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The Metabolic Syndrome and Chronic Kidney Disease in U.S. Adults. Ann Intern Med. 2004;140(3):167. DOI: https://doi.org/10.7326/0003-4819-140-3-200402030-00007
9. Kim JS, Hwang HS. Vascular Calcification in Chronic Kidney Disease: Distinct Features of Pathogenesis and Clinical Implication. Korean Circ J. 2021;51(12):961–982. DOI: https://doi.org/10.4070/kcj.2021.0995
10. Hwang HS, Cho JS, Hong YA, Chang YK, Kim SY, Shin SJ, Yoon HE. Vascular calcification and left ventricular hypertrophy in hemodialysis patients: interrelationship and clinical impacts. Int J Med Sci. 2018;15(6):557–563. DOI: https://doi.org/10.7150/ijms.23700
11. López B, González A, Hermida N, Laviades C, Díez J. Myocardial fibrosis in chronic kidney disease: potential benefits of torasemide. Kidney Int. 2008;74:S19–23. DOI: https://doi.org/10.1038/ki.2008.512
12. Moe SM, Kulkarni DP. Disorders of Calcium, Phosphorus, and Magnesium Homeostasis. In: National Kidney Foundation’ s Primer on Kidney Diseases [Internet]. Elsevier; 2018 [cited 2024 Dec 5]. p. 107–19. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780323477949000111 DOI: https://doi.org/10.1016/B978-0-323-47794-9.00011-1
13. Adhami M, Ghori-Javed FY, Chen H, Gutierrez SE, Javed A. Runx2 Regulates the Gene Network Associated with Insulin Signaling and Energy Homeostasis. Cells Tissues Organs. 2011;194(2–4):232–237. DOI: https://doi.org/10.1159/000324763
14. Dörr K, Kammer M, Reindl-Schwaighofer R, Lorenz M, Prikoszovich T, Marculescu R, Beitzke D, Wielandner A, Erben RG, Oberbauer R. Randomized Trial of Etelcalcetide for Cardiac Hypertrophy in Hemodialysis. Circ Res. 2021;128(11):1616–1625. DOI: https://doi.org/10.1161/CIRCRESAHA.120.318556
15. Nakano T, Kishimoto H, Tokumoto M. Direct and indirect effects of fibroblast growth factor 23 on the heart. Front Endocrinol. 2023;14:1059179. DOI: https://doi.org/10.3389/fendo.2023.1059179
16. Raaz U, Schellinger IN, Chernogubova E, Warnecke C, Kayama Y, Penov K, Hennigs JK, Salomons F, Eken S, Emrich FC, Zheng WH, Adam M, Jagger A, Nakagami F, Toh R, Deng A, Buerke M, Maegdefessel L, Hasenfuß G. Transcription Factor Runx2 Promotes Aortic Fibrosis and Stiffness in Type 2 Diabetes Mellitus. Circ Res. 2015;117(6):513–524. DOI: https://doi.org/10.1161/CIRCRESAHA.115.306341
17. Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol. 2019;15(10):569–589. DOI: https://doi.org/10.1038/s41574-019-0242-2
18. Luo T, Nocon A, Fry J, Sherban A, Rui X, Jiang B, Xu XJ, Han J, Yan Y, Yang Q, Li Q. AMPK Activation by Metformin Suppresses Abnormal Extracellular Matrix Remodeling in Adipose Tissue and Ameliorates Insulin Resistance in Obesity. Diabetes. 2016;65(8):2295–2310. DOI: https://doi.org/10.2337/db15-1122
19. Wu M, Xu H, Liu J, Tan X, Wan S, Guo M, Long Y, Sugawara A. Metformin and Fibrosis: A Review of Existing Evidence and Mechanisms. Sugawara A, editor. J Diabetes Res. 2021;2021:1–11. DOI: https://doi.org/10.1155/2021/6673525
20. Biondo LA, Batatinha HA, Souza CO, Teixeira AAS, Silveira LS, Alonso-Vale MI, Oyama LM, Alves MJ, Seelaender M, Neto JCR. Metformin Mitigates Fibrosis and Glucose Intolerance Induced by Doxorubicin in Subcutaneous Adipose Tissue. Front Pharmacol. 2018;9:452. DOI: https://doi.org/10.3389/fphar.2018.00452
21. Chieng D, Kistler PM. Coffee and tea on cardiovascular disease (CVD) prevention. Trends Cardiovasc Med. 2022;32(7):399–405. DOI: https://doi.org/10.1016/j.tcm.2021.08.004
22. Rohman MS, Lukitasari M, Adi Nugroho D, Nashi W, Ida Panca Nugraheini N, Wahyu Sardjono E. Development of an Experimental Model of Metabolic Syndrome in Sprague Dawley Rat. Res J Life Sci. 2017 Apr 1;4(1):76–86. DOI: https://doi.org/10.21776/ub.rjls.2017.004.01.10
23. Nugroho DA, Lukitasari M, Marlita M, Rohman MS, Widodo N, Kusumastuty I, et al. Dose-dependent Decaffeinated Green Tea Extract Administration Improved Hyperglycemia through Modulation of IRS-1 and GLUT-4 Genes Expression in Metabolic Syndrome Rat Model: In: Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia [Internet]. Purwokerto, Indonesia: SCITEPRESS - Science and Technology Publications; 2020 [cited 2024 Dec 5]. p. 69–74. Available from: https://www.scitepress.org/DigitalLibrary/Link.aspx?doi=10.5220/0010487900690074 DOI: https://doi.org/10.5220/0010487900690074
24. Lukitasari M, Rohman MS, Nugroho DA, Wahyuni NA, Nur Kholis M, Widodo N. Improvement of Cardiac Fibrosis Biomarkers through Inflammation Inhibition by Green Tea and Decaffeinated Light Roasted Green Coffee Extract Combination Administration in Metabolic Syndrome Rat Model. F1000Research. 2021;10:1013. DOI: https://doi.org/10.12688/f1000research.55468.1
25. Lukitasari M, Nugroho DA, Rohman MS. Green Tea Extract Administration had a Beneficial Effect on PPAR Alpha And PPAR Gamma Gene Expression in Metabolic Syndrome Rat Model. J Hypertens. 2018 Jul;36(Supplement 2):e9. DOI: https://doi.org/10.1097/01.hjh.0000544393.97442.09
26. Wang X, Xu Z, Chang R, Zeng C, Zhao Y. High-Fructose Diet Induces Cardiac Dysfunction via Macrophage Recruitment in Adult Mice. J Cardiovasc Pharmacol Ther. 2023 Jan 1;28:10742484231162249. DOI: https://doi.org/10.1177/10742484231162249
27. Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res. 2021;117(6):1450–1488. DOI: https://doi.org/10.1093/cvr/cvaa324
28. Dai Z, Aoki T, Fukumoto Y, Shimokawa H. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J Cardiol. 2012;60(5):416–421. DOI: https://doi.org/10.1016/j.jjcc.2012.06.009
29. Nascimento AR, Machado M, De Jesus N, Gomes F, Lessa MA, Bonomo IT, Tibiriçá E. Structural and functional microvascular alterations in a rat model of metabolic syndrome induced by a high‐fat diet. Obesity. 2013;21(10):2046–54. DOI: https://doi.org/10.1002/oby.20358
30. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Fieldman HI, St. John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-o M, Kusek JW, Keane MG, Wolf M.. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011 Nov;121(11):4393–4408. DOI: https://doi.org/10.1172/JCI46122
31. Van Der Vaart A, Yeung SMH, Van Dijk PR, Bakker SJL, De Borst MH. Phosphate and fibroblast growth factor 23 in diabetes. Clin Sci. 2021;135(14):1669–1687. DOI: https://doi.org/10.1042/CS20201290
32. Zhang X, Guo K, Xia F, Zhao X, Huang Z, Niu J. FGF23C-tail improves diabetic nephropathy by attenuating renal fibrosis and inflammation. BMC Biotechnol. 2018;18(1):33. DOI: https://doi.org/10.1186/s12896-018-0449-7
33. De Las Rivas M, Lira-Navarrete E, Gerken TA, Hurtado-Guerrero R. Polypeptide GalNAc-Ts: from redundancy to specificity. Curr Opin Struct Biol. 2019;56:87–96. DOI: https://doi.org/10.1016/j.sbi.2018.12.007
34. Takashi Y, Fukumoto S. FGF23 beyond Phosphotropic Hormone. Trends Endocrinol Metab. 2018;29(11):755–767. DOI: https://doi.org/10.1016/j.tem.2018.08.006
35. Cheng F, Hulley P. The osteocyte—A novel endocrine regulator of body phosphate homeostasis. Maturitas. 2010;67(4):327–338 DOI: https://doi.org/10.1016/j.maturitas.2010.08.011
36. Wang Y kai, Li S jie, Zhou L lu, Li D, Guo L wei. GALNT3 protects against vascular calcification by reducing oxidative stress and apoptosis of smooth muscle cells. Eur J Pharmacol. 2023:939:175447. DOI: https://doi.org/10.1016/j.ejphar.2022.175447
37. Choe M, Brusgard JL, Chumsri S, Bhandary L, Zhao XF, Lu S, Goloubeva OG, Polster BM, Fiskum GR, Girnun GD, Kim MS, Passaniti A. The RUNX2 Transcription Factor Negatively Regulates SIRT6 Expression to Alter Glucose Metabolism in Breast Cancer Cells. J Cell Biochem. 2015;116(10):2210–2226. DOI: https://doi.org/10.1002/jcb.25171
38. Gupta RC, Chang D, Nammi S, Bensoussan A, Bilinski K, Roufogalis BD. Interactions between antidiabetic drugs and herbs: an overview of mechanisms of action and clinical implications. Diabetol Metab Syndr. 2017;9:59. DOI: https://doi.org/10.1186/s13098-017-0254-9
39. Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr. 2003;78(4):728–733. DOI: https://doi.org/10.1093/ajcn/78.4.728
40. Yu C, Jiao Y, Xue J, Zhang Q, Yang H, Xing L, Chen G, Wu J, Zhang S, Zhu W, Cao J. Metformin Sensitizes Non-small Cell Lung Cancer Cells to an Epigallocatechin-3-Gallate (EGCG) Treatment by Suppressing the Nrf2/HO-1 Signaling Pathway. Int J Biol Sci. 2017;13(12):1560–1569. DOI: https://doi.org/10.7150/ijbs.18830
41. Phadwal K, Koo E, Jones RA, Forsythe RO, Tang K, Tang Q, Corcoran BM, Caporali A, MacRae VE.. Metformin protects against vascular calcification through the selective degradation of Runx2 by the p62 autophagy receptor. J Cell Physiol. 2022;237(11):4303–4316. DOI: https://doi.org/10.1002/jcp.30887
42. Zhang SL, Chen ZH, Lin DT, Yan Q, Gao F, Lin H. Epigallocatechin gallate regulates inflammatory responses and new bone formation through Wnt/β-Catenin/COX-2 pathway in spondyloarthritis. Int Immunopharmacol. 2021;98:107869. DOI: https://doi.org/10.1016/j.intimp.2021.107869
43. Kozlov AV, Javadov S, Sommer N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants. 2024;13(5):602. DOI: https://doi.org/10.3390/antiox13050602
44. Chen K, Chen W, Liu SL, Wu TS, Yu KF, Qi J, et al. Epigallocatechingallate attenuates myocardial injury in a mouse model of heart failure through TGF‑β1/Smad3 signaling pathway. Mol Med Rep. 2018;17(6):7652-7660. DOI: https://doi.org/10.3892/mmr.2018.8825
45. Cui Y, Wang Y, Liu G. Epigallocatechin gallate (EGCG) attenuates myocardial hypertrophy and fibrosis induced by transverse aortic constriction via inhibiting the Akt/mTOR pathway. Pharm Biol. 2021;59(1):1303–1311. DOI: https://doi.org/10.1080/13880209.2021.1972124
46. Chomsy IN, Rohman MS, Khotimah H, Bramantyo BB, Auzan A, Lukitasari M, Nugroho DA. Effect of the ethanolic extract of green tea and green coffee on cardiac fibrosis attenuation by suppressing activin-a and collagen-1 gene expression. In Gowa, Indonesia; 2022 [cited 2024 Dec 5]. p. 020002. Available from: https://pubs.aip.org/aip/acp/article/2827624 DOI: https://doi.org/10.1063/5.0099004
47. Suryono S, Amien MI, Tohari AI, Saputra AD, Hidayat MRF, Ramadhan HF. Effect of Moringa oleifera Leaf Extract on TGF-β1 and Galectin-3 Levels and Cardiac Fibrosis in Diabetic Rat. TJNPR. 2024;8(11):8988-8992. DOI: https://doi.org/10.26538/tjnpr/v8i11.4
48. Firdausi SR, Nur'aini RAR, Izzah FN, Nabilah SN, Christina YI, Dwijayanti DR, Rahayu S, Djati MS. Elephantopus scaber Ethanol Extract Suppresses Inflammation via Regulation of the NF-κB Pathway Expression in Pulmonary Fibrosis. TJNPR. 2024;8(9):8554-8560. DOI: https://doi.org/10.26538/tjnpr/v8i9.44