Modulation of Cytokine Imbalance by Phaleria macrocarpa Leaf Extract in LPS-stimulated RAW 264.7 Macrophages
Main Article Content
Abstract
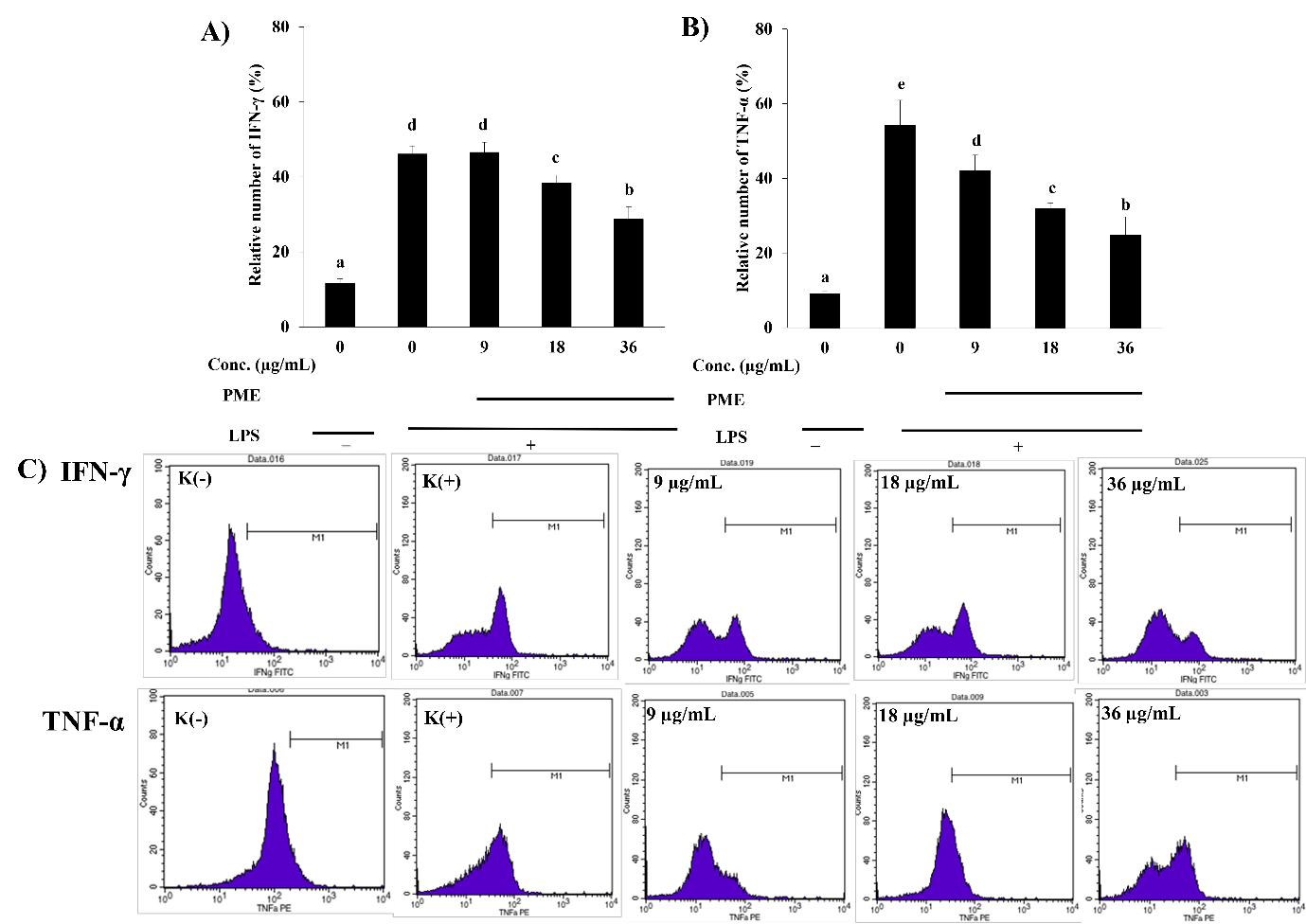
Chronic inflammation is strongly associated with excessive production of pro-inflammatory cytokines. Mahkota Dewa (Phaleria macrocarpa) is a traditional medicinal plant recognized for its anti-inflammatory potential. Nevertheless, its specific role in modulating pro- and anti-inflammatory cytokines within immune cells remains inadequately explored. This study aimed to investigate the anti-inflammatory activity of P. macrocarpa leaf extract by assessing its regulatory effects on pro-inflammatory (IFN-γ and TNF-α) and anti-inflammatory cytokines (IL-10 and TGF-β) in RAW 264.7 macrophage cells stimulated with lipopolysaccharide (LPS). The cells were treated with P. macrocarpa leaf extract at 9, 18, and 36 µg/mL, none of which exhibited cytotoxic effects. Cytokine levels were measured using flow cytometry. The findings demonstrated a significant reduction (p<0.05) in IFN-γ and TNF-α levels at 18 and 36 µg/mL of P. macrocarpa leaf extract. Furthermore, IL-10 levels increased in a dose-dependent manner, while the highest TGF-β expression was observed at 18 µg/mL. The findings revealed that P. macrocarpa leaf extract exerts significant anti-inflammatory effects by modulating cytokine production in LPS-induced macrophages. These results suggest its broader therapeutic potential in managing inflammation-related diseases, warranting further molecular and clinical investigation.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019; 20(23):1-31. Doi:10.3390/ijms20236008. DOI: https://doi.org/10.3390/ijms20236008
2. Al-Qahtani AA, Alhamlan FS, Al-Qahtani AA. Pro-Inflammatory and anti-inflammatory interleukins in infectious diseases: A comprehensive review. Trop Med Infect Dis. 2024; 9(1):1-20. Doi: 10.3390/tropicalmed9010013. DOI: https://doi.org/10.3390/tropicalmed9010013
3. Mohammadi F, Rahimi K, Ahmadi A, Hooshmandi Z, Amini S, Mohammadi A. Anti-inflammatory effects of Mentha pulegium L. extract on human peripheral blood mononuclear cells are mediated by TLR-4 and NF-κB suppression. Heliyon. 2024; 10(1):1-16. Doi: 10.1016/j.heliyon.2024.e24040 DOI: https://doi.org/10.1016/j.heliyon.2024.e24040
4. Ngo VTH, Bajaj T. Ibuprofen. [Online]. 2025 [Cited 2024 Aug 11]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542299/
5. Skriver C, Maltesen T, Dehlendorff C, Skovlund CW, Schmidt M, Sørensen HT, Friis S. Long-term aspirin use and cancer risk: a 20-year cohort study. J Natl Cancer Inst. 2024; 116(4):530-538. Doi: 10.1093/jnci/djad231 DOI: https://doi.org/10.1093/jnci/djad231
6. Traserra S, Barber C, Alcalá-González LG, Landolfi S, Lange R,Malagelada C, Corsetti M, Jimenez M. Evaluation of the mechanism of action of paracetamol, drotaverine, and peppermint oil and their effects in combination with hyoscine butylbromide on colonic motility: human ex-vivo study. Front Pharmacol. 2024; 15:1384070. Doi: 10.3389/fphar.2024.1384070 DOI: https://doi.org/10.3389/fphar.2024.1384070
7. Sohail R, Mathew M, Patel KK, Reddy SA, Haider Z, Naria M, Habib A, Abdin ZU, Razzaq Chaudhry W, Akbar A. Effects of Non-steroidal Anti-inflammatory Drugs (NSAIDs) and Gastroprotective NSAIDs on the gastrointestinal tract: A narrative review. Cureus. 2023; 15(4):1-14. Doi:10.7759/cureus.37080 DOI: https://doi.org/10.7759/cureus.37080
8. Okolie NP, Falodun A, Davids O. Evaluation of the antioxidant activity of root extract of pepper fruit (Dennetia tripetala), and it's potential for the inhibition of lipid peroxidation. Afr J Tradit Complement Altern Med. 2014; 11(3):221-227. doi: 10.4314/ajtcam.v11i3.31. DOI: https://doi.org/10.4314/ajtcam.v11i3.31
9. Dwijayanti DR, Widyananda MH, Hermanto FE, Soewondo A, Afiyanti M, Widodo N. Revealing the anti-inflammatory activity of Euphorbia hirta extract: transcriptomic and nitric oxide production analysis in LPS-induced RAW 264.7 cells. Food Agric Immunol. 2024; 35(1):1-15. Doi: 10.1080/09540105.2024.2351360 DOI: https://doi.org/10.1080/09540105.2024.2351360
10. Djati MS, Christina YI. Traditional Indonesian rempah-rempah as a modern functional food and herbal medicine. Funct Foods Health Dis. 2019; 9(4):241-264. Doi: 10.31989/ffhd.v9i4.571. DOI: https://doi.org/10.31989/ffhd.v9i4.571
11. Christina YI, Rifa’i M, Widodo N, Djati MS. Comparative study of antiproliferative activity in different plant parts of Phaleria macrocarpa and the underlying mechanism of action. Sci World J. 2022; 2022(1):1-13. Doi: 10.1155/2022/3992660 DOI: https://doi.org/10.1155/2022/3992660
12. Alara OR, Alara JA, Olalere OA. Review on Phaleria macrocarpa pharmacological and phytochemical properties. Drug Des. 2016; 5(3):2169-0138. Doi: 10.4172/2169-0138.1000134 DOI: https://doi.org/10.4172/2169-0138.1000134
13. Hendra R, Ahmad S, Oskoueian E, Sukari A, Shukor MY. Antioxidant, anti-inflammatory and cytotoxicity of Phaleria macrocarpa (Boerl.) Scheff Fruit. BMC Complement Altern Med. 2011; 11:1-10. doi: 10.1186/1472-6882-11-110. DOI: https://doi.org/10.1186/1472-6882-11-110
14. Christina YI, Nafisah W, Widodo, Rifa’i M, Djati MS. Evaluation of total phenolic, flavonoid contents, antioxidant and cytotoxicity activities of various parts of Phaleria macrocarpa (Scheff.) Boerl fruit. IOP Conf Ser: Earth Environ Sci. 2021; 743:1-7. Doi: 10.1088/1755-1315/743/1/012026 DOI: https://doi.org/10.1088/1755-1315/743/1/012026
15. Christina YI, Nafisah W, Widodo, Rifa’i M, Djati MS. In vitro antioxidant and anticancer activity of crude ethanol extract of Mahkota Dewa (Phaleria macrocarpa) leaves. AIP Conf Proc. 2021; 2353:030015-1–030015-5. Doi: 10.1063/5.0052692 DOI: https://doi.org/10.1063/5.0052692
16. Lestari IC, Lindarto D, Ilyas S, Widyawati T, Mustofa, Jusuf NK, Hasibuan PAZ, Siahaan L. Characterization and Antioxidant Activity of Phaleria macrocarpa (Scheff.) Boerl Leaf Ethanol Extract. In: IOP Conference Series: Earth and Environmental Science. IOP Publishing. 2023; 1188(1):012046 DOI: https://doi.org/10.1088/1755-1315/1188/1/012046
17. Aminullah L, Christina YI, Rifa’i M, Djati MS. Phaleria macrocarpa leaves extract reduce tumors growth and improve histological changes of liver and kidney on 4T1 breast cancer mice model. J Exp Life Sci. 2022; 2(2):46-54. Doi: 10.21776/ub.jels.2022.012.02.02 DOI: https://doi.org/10.21776/ub.jels.2022.012.02.02
18. Christina YI, Nafisah W, Atho’illah MF, Rifa’i M, Widodo N, Djati MS. Anti-breast cancer potential activity of Phaleria macrocarpa (Scheff.) Boerl. leaf extract through in silico studies. J Pharm Pharmacogn Res. 2021; 9(6):824-845. Doi: 10.56499/jppres21.1092_9.6.824 DOI: https://doi.org/10.56499/jppres21.1092_9.6.824
19. Kusmardi K, Situmorang NY, Zuraidah E, Estuningtyas A, Tedjo A. The effect of Mahkota Dewa (Phaleria macrocarpa) leaf extract on the Mucin 1 expression in mice colonic epithelial cells induced by Dextran Sodium Sulfate (DSS). Pharmacogn J. 2021; 13(6):1509-1515. Doi: 10.5530/pj.2021.13.192 DOI: https://doi.org/10.5530/pj.2021.13.192
20. Lestari IC, Lindarto D, Ilyas S, Widyawati T, Mustofa, Jusuf NK, Hasibuan PAZ, Siahaan L, Rusda M, Amin MM. Effect
21. of Phaleria Macrocarpa (Scheff.) Boerl leaf ethanol extract on serum IL-6 and TNF-α levels in diabetic rats. Med Arch. 2023; 77(4):254-257. doi: 10.5455/medarh.2023.77.254-257. DOI: https://doi.org/10.5455/medarh.2023.77.254-257
22. Djati MS, Christina YI, Dwijayanti DR, Rahayu S, Djati MS. Synergistic modulation of proinflammatory mediators and cytokines in lipopolysaccharide-activated RAW 264.7 macrophages: The therapeutic potential of Elephantopus scaber and Sauropus androgynus ethanol extract. Vet World. 2024; 17(3):728-734. doi: 10.14202/vetworld.2024.728-734 DOI: https://doi.org/10.14202/vetworld.2024.728-734
23. Shofiah A, Christina YI, Dwijayanti DR, Soewondo A, Widodo N, Djati MS. Exploring the inflammatory pathway modulation of Phaleria macrocarpa: evidence from in vitro and in silico studies. Pharmacia. 2025; 72:1-14. doi: 10.3897/pharmacia.72.e153095. DOI: https://doi.org/10.3897/pharmacia.72.e153095
24. Naseri N, Kalantar K, Amirghofran Z. Anti-inflammatory activity of Echium amoenum extract on macrophages mediated by inhibition of inflammatory mediators and cytokines expression. Res Pharm Sci. 2018; 13(1):73-81. doi: 10.4103/1735-5362.220970. DOI: https://doi.org/10.4103/1735-5362.220970
25. Ha Y, Lee WH, Jeong JW, Park M, Ko JY, Kwon OW, Le J, Kim JY. Pyropia yezoensis Extract Suppresses IFN-Gamma- and TNF-Alpha-Induced Proinflammatory Chemokine Production in HaCaT Cells via the Down-Regulation of NF-Κb. Nutrients. 2020;12(5):1238 DOI: https://doi.org/10.3390/nu12051238
26. Kopitar-Jerala N. The Role of Interferons in Inflammation and Inflammasome Activation. Front Immunol. 2017; 8:1-9. doi: 10.3389/fimmu.2017.00873. DOI: https://doi.org/10.3389/fimmu.2017.00873
27. Yu J, Wei M, Becknell B, Trotta R, Liu S, Boyd Z, Jaung MS, Blaser BW, Sun J, Benson DM Jr, Mao H, Yokohama A, Bhatt D, Shen L, Davuluri R, Weinstein M, Marcucci G, Caligiuri MA. Pro- and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity. 2006; 24(5):575-90. doi: 10.1016/j.immuni.2006.03.016. DOI: https://doi.org/10.1016/j.immuni.2006.03.016
28. Haryana SM, Sismindari, Soesatyo MGM. The effect of Mahkota Dewa (Phaleria macrocarpa (Scheff) Boerl) leaf etanolic extract on splenic NK 1.1 cells activity. J Med Sci. 40(3):1-7.
29. Chen L, Huang Z, Liao Y, Yang B, Zhang J. Association between tumor necrosis factor polymorphisms and rheumatoid arthritis as well as systemic lupus erythematosus: a meta-analysis. Braz J Med Biol Res. 2019; 52(3):1-9. doi: 10.1590/1414-431X20187927. DOI: https://doi.org/10.1590/1414-431x20187927
30. Ghorbaninezhad F, Leone P, Alemohammad H, Najafzadeh B, Nourbakhsh NS, Prete M, Malerba E, Saeedi H, Tabrizi NJ, Racanelli V, Baradaran B. Tumor necrosis factor‑α in systemic lupus erythematosus: Structure, function and therapeutic implications (Review). Int J Mol Med. 2022; 49(4):1-13. doi: 10.3892/ijmm.2022.5098. DOI: https://doi.org/10.3892/ijmm.2022.5098
31. Kusmardi K, Ramadhoani SR, Estuningtyas ARI. Anti-inflammatory effect of Mahkota Dewa (Phaleria macrocarpa) leaf extract loaded in chitosan nanoparticles in reducing tumor necrosis factor α expression on colon of dextran sodium sulfat-induced mice. Int J Pharm Res. 2019; 11:624-631. DOI: https://doi.org/10.22159/ajpcr.2018.v11i6.24159
32. Carlini V, Noonan DM, Abdalalem E, Goletti D, Sansone C, Calabrone L, Albini A. The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front Immunol. 2023; 14:1-19. doi: 10.3389/fimmu.2023.1161067. DOI: https://doi.org/10.3389/fimmu.2023.1161067
33. Kessler B, Rinchai D, Kewcharoenwong C, Nithichanon A, Biggart R, Hawrylowicz CM, Bancroft GJ, Lertmemongkolchai G. Interleukin 10 inhibits pro-inflammatory cytokine responses and killing of Burkholderia pseudomallei. Sci Rep. 2017;7:1-11. doi: 10.1038/srep42791. DOI: https://doi.org/10.1038/srep42791
34. Alexander AF, Forbes H, Miller-Jensen K. Single-cell secretion analysis reveals a dual role for IL-10 in restraining and resolving the TLR4-induced inflammatory response. Cell reports. 2021; 36(12):109728. DOI: 10.1016/j.celrep.2021.109728 DOI: https://doi.org/10.1016/j.celrep.2021.109728
35. Zhang F, Wang H, Wang X, Jiang G, Liu H, Zhang G, Wang H, Fang R, Bu X, Cai S, Du J. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016; 7(32):52294-52306. doi: 10.18632/oncotarget.10561. DOI: https://doi.org/10.18632/oncotarget.10561
36. Gauthier T, Yao C, Dowdy T, Jin W, Lim YJ, Patiño LC, Liu N, Ohlemacher SI, Bynum A, Kazmi R, Bewley CA, Mitrovic M, Martin D, Morell RJ, Eckhaus M, Larion M, Tussiwand R, O'Shea JJ, Chen W. TGF-β uncouples glycolysis and inflammation in macrophages and controls survival during sepsis. Sci Signal. 2023;16(797):1-35. doi: 10.1126/scisignal.ade0385. DOI: https://doi.org/10.1126/scisignal.ade0385
37. Mirzoeva S, Franzen CA, Pelling JC. Apigenin inhibits TGF-β-induced VEGF expression in human prostate carcinoma cells via a Smad2/3- and Src-dependent mechanism. Mol Carcinog. 2014; 53(8):598-609. doi: 10.1002/mc.22005. DOI: https://doi.org/10.1002/mc.22005
38. Yang HW, Kim HJ, Park, JH, Shin JM, Lee HM. Apigenin alleviates TGF-β1-induced nasal mucosa remodeling by inhibiting MAPK / NF-kB signaling pathways in chronic rhinosinusitis. PLOS One. 2018; 13(8);0201595. DOI: 10.1371/journal.pone.020159 DOI: https://doi.org/10.1371/journal.pone.0201595
39. Li LH, Lu B, Wu HK, Zhang H, Yao FF. Apigenin inhibits TGF-β1-induced proliferation and migration of airway smooth muscle cells. Int J Clin Exp Pathol. 2015; 8(10):12557-12563.