Phytochemical Profiling of Limau Kuit (Citrus jambhiri Lush.) using FTIR Spectroscopy and Multivariate Analysis
Main Article Content
Abstract
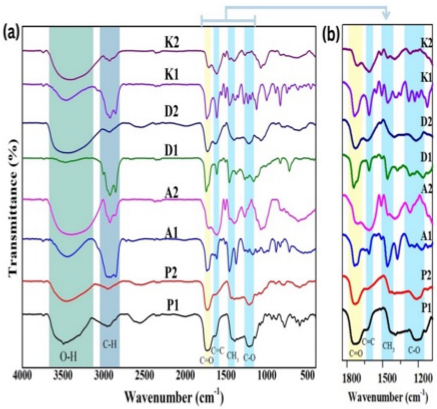
Citrus jambhiri Lush., locally known as Limau Kuit, is an indigenous citrus species from South Kalimantan, Indonesia, traditionally used by the Banjar community for food and medicinal purposes. This study aimed to characterize the phytochemical composition of various parts (leaves, fruit peel, fruit pulp, and fruit juice) of C. jambhiri using Fourier Transform Infrared (FTIR) spectroscopy combined with principal component analysis (PCA). Extractions were performed using three solvents of varying polarity: n-hexane, ethyl acetate, and ethanol. The phytochemical profile of the extracts was characterized by Fourier Transform Infrared (FTIR) spectroscopy followed by multivariate data analysis with chemometric techniques. FTIR spectra revealed the presence of key functional groups such as O–H, C–H, C=O, and C=C, suggesting the existence of secondary metabolites, including flavonoids, terpenoids, and saponins. Chemometric analysis using principal component analysis (PCA) and cluster analysis showed that solvent polarity had a greater impact on chemical composition than plant part, with clear clustering observed among extracts prepared using the same solvent. Juice extracts formed a distinct cluster, indicating the presence of unique compounds compared to other parts. These findings underscore the importance of solvent selection in targeting specific bioactive constituents from C. jambhiri. The study provides a scientific basis for the potential development of C. jambhiri-based functional food ingredients and herbal formulations and highlights the value of FTIR–PCA as a rapid, non-destructive method for phytochemical profiling.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Nugroho HYSH, Nurfatriani F, Indrajaya Y, Yuwati TW, Ekawati S, Salminah M, Gunawan H, Subarudi S, Sallata MK, Allo MK, Muin N, Isnan W, Putri IASLP, Prayudyaningsih R, Ansari F, Siarudin M, Setiawan O, Baral H. Mainstreaming ecosystem services from Indonesia’s remaining forests. Sustainability. 2022; 14(19):1-39. Doi:10.3390/su141912124 DOI: https://doi.org/10.3390/su141912124
2. Purnama A, Mardina V, Puspita K, Qanita I, Rizki DR, Hasballah K, Iqbal M, Sarong M. Molecular docking of two cytotoxic compounds from Calotropis gigantea leaves against therapeutic molecular target of pancreatic cancer. Narra J. 2021; 1(2):1-9. Doi:10.52225/narraj.v1i2.37 DOI: https://doi.org/10.52225/narraj.v1i2.37
3. Ariyanthini KS, Angelina E, Andina NKDP, Wijaya H, Wiratama IPRKP, Naripradnya PS, Putra IGAND, Setyawan EI. Implementation of principal component analysis-cluster analysis on the extraction of Green Tea Leaf (Camellia sinensis (L.) Kuntze). Biointerface Res Appl Chem. 2022; 13(4):1-14. Doi:10.33263/BRIAC134.335 DOI: https://doi.org/10.33263/BRIAC134.335
4. Irwan A, Mustikasari K, Ariyani D. Chemical preliminary evaluation of leaves, peels, and fleshs fruit of Limau Kuit: local orange of South Kalimantan. J Sains Terap Kim. 2017; 11(2):71-79. Doi:10.20527/jstk.v11i2.4040 DOI: https://doi.org/10.20527/jstk.v11i2.4040
5. Buih PTJ and Susandarini R. Morphological characterization of Citrus jambhiri Lush. and its kinship with Citrus amblycarpa (Hassk.) Ochse. Al-Kauniyah J Biol. 2023; 16(2):255-268. Doi:10.15408/kauniyah.v16i2.22920 DOI: https://doi.org/10.15408/kauniyah.v16i2.22920
6. Kasman K, Ishak NI, Hastutiek P, Suprihati E, Mallongi A. Identification of active compounds of ethanol extract of Citrus amblycarpa leaves by analysis of thin-layer chromatography and gas chromatography-mass spectrometry as bioinsecticide candidates for mosquitoes. Open Access Maced J Med Sci. 2020; 8(T2):1-6. Doi:10.3889/OAMJMS.2020.5207 DOI: https://doi.org/10.3889/oamjms.2020.5207
7. Saragih WS, Purba E, Lisnawita L, Basyuni M. The Fourier transform infrared spectroscopy from Diplazium esculentum and Rivina humilis analysis to reveals the existence of necessary components in oil palm plantations of Ganoderma boninense control. Biodiversitas J Biol Divers. 2021; 22(9):3654-3660. Doi:10.13057/biodiv/d220902 DOI: https://doi.org/10.13057/biodiv/d220902
8. Dewi FRP, Lim V, Rosyidah A, Fatimah F, Wahyuningsih SPA, Zubaidah U. Characterization of silver nanoparticles (AgNPs) synthesized from Piper ornatum leaf extract and its activity against food borne pathogen Staphylococcus aureus. Biodiversitas J Biol Divers. 2023; 24(3):1742-1748. Doi:10.13057/biodiv/d240348 DOI: https://doi.org/10.13057/biodiv/d240348
9. Siti Dharmawati, Aam Gunawan, Antoni Pardede, Osfar Sjofjan. Characteristics of natural collagen of freshwater Snail Flesh (Pomacea paludosa) extracted with bromelain enzyme and acid-hydro-extraction method. Trop J Nat Prod Res. 2025; 9(3):944-941. Doi:10.26538/tjnpr/v9i3.7 DOI: https://doi.org/10.26538/tjnpr/v9i3.7
10. Ermawati, Ahmad A, Sartini S, Hasan N, Permana AD, Leman Y, Duppa MT, Karim H, Fajriah S, Sapar A, Atun S. Evaluation of antidiabetic effects of Watermelon rind Extract: Integrative computational simulations and in vitro studies. Trop J Nat Prod Res. 2024; 8(10):8629-8639. Doi:10.26538/tjnpr/v8i10.3 DOI: https://doi.org/10.26538/tjnpr/v8i10.3
11. Giorgini E, Notarstefano V, Foligni R, Carloni P, Damiani E. First ATR-FTIR Characterization of Black, Green and White Teas (Camellia sinensis) from European Tea Gardens: A PCA Analysis to Differentiate Leaves from the In-Cup Infusion. Foods. 2024; 13(1):1-14. Doi:10.3390/foods13010109 DOI: https://doi.org/10.3390/foods13010109
12. Rohman A, Arifah FH, Alam G, Muchtaridi M. The application of FTIR spectroscopy and chemometrics for classification of Mangosteen extract and its correlation with alpha-mangostin. J Appl Pharm Sci. 2020; 10(4):149-154. Doi:10.7324/JAPS.2020.104019 DOI: https://doi.org/10.7324/JAPS.2020.104019
13. Umar AH, Syahruni R, Ranteta’dung I, Rafi M. FTIR-based fingerprinting combined with chemometrics method for rapid discrimination of Jatropha spp. (Euphorbiaceae) from different regions in South Sulawesi. J Appl Pharm Sci. 2023; 13(1):139-149. Doi:10.7324/JAPS.2023.130113 DOI: https://doi.org/10.7324/JAPS.2023.130113
14. Karta IW, Warsito W, Masruri M, Mudianta IW. Effects of solvent polarity on phytoconstituents, antioxidant and anti- inflammatory activities of Dracaena angustifolia roxb root bark extracts. Trop J Nat Prod Res. 2024; 8(5):7148-7153. Doi:10.26538/tjnpr/v8i5.15 DOI: https://doi.org/10.26538/tjnpr/v8i5.15
15. Retnosari D, Purnobasuki H, Supriyanto A. Antimalarial activity of Crude bark extract of Pterocarpus indicus Willd. against Plasmodium falciparum strain 3D7. Trop J Nat Prod Res. 2023; 7(9):3893-3897. Doi:10.26538/tjnpr/v7i9.6 DOI: https://doi.org/10.26538/tjnpr/v7i9.6
16. Guntarti A, Salamah N, Fadli J. Authentication of commercial corn oil products using combined GC-MS and FTIR chemometric methods. Trop J Nat Prod Res. 2025; 9(4):1513-1518. Doi:10.26538/tjnpr/v9i4.20 DOI: https://doi.org/10.26538/tjnpr/v9i4.20
17. Sumarlin LO, Heryanto R, Karomah AH, Nurcholis W. UHPLC-HRMS-Based metabolomics to evaluate the antibacterial compounds of Coix lacryma-jobi seeds with different extraction solvent concentrations. Trop J Nat Prod Res. 2024; 8(8):8087-8092. Doi:10.26538/tjnpr/v8i8.24 DOI: https://doi.org/10.26538/tjnpr/v8i8.24
18. Syafri S, Hafiz A, Syofyan S, Alen Y, Hamidi D. FT-IR fingerprinting analysis for classification of West Sumatra small ginger (Zingiber officinale Roscoe) essential oil and its antioxidant activity. Trop J Nat Prod Res. 2024; 8(2):6081-6086. Doi:10.26538/tjnpr/v8i2.4 DOI: https://doi.org/10.26538/tjnpr/v8i2.4
19. Lestari NB, Sulistyaningsih YC, Umar AH, Ratnadewi D. Distribution and FTIR-based fingerprint of secondary metabolites in different organs of ant-plant (Myrmecodia tuberosa). Biodiversitas J Biol Divers. 2024; 25(3):1104-1115. Doi:10.13057/biodiv/d250325 DOI: https://doi.org/10.13057/biodiv/d250325
20. Wulandari R, Sudjadi, Martono S, Rohman A. Liquid Chromatography and Fourier Transform Infrared Spectroscopy for quantitative analysis of individual and total curcuminoid in Curcuma longa extract. J Appl Pharm Sci. 2018; 8(9):107-113. Doi:10.7324/JAPS.2018.8916 DOI: https://doi.org/10.7324/JAPS.2018.8916
21. Mulla MFZ, Ahmed J, Vahora A, Pathania S, Rashed MS. Characterization of biopolymers based antibacterial films enriched with thyme essential oil and their application for milk cake preservation. Front Food Sci Technol. 2024; 4:1-13. Doi:10.3389/frfst.2024.1356582 DOI: https://doi.org/10.3389/frfst.2024.1356582
22. Abdul R, Riana L, Fajar Aji L. Near infrared (NIR) and fourier transform infrared (FTIR) spectroscopy combined with chemometrics for agrifood products analysis. In: Chemometrics. Elsevier. 2024. 125-146 pp. Doi:10.1016/B978-0-443-21493-6.00006-X. DOI: https://doi.org/10.1016/B978-0-443-21493-6.00006-X
23. Salamah N, Cantika CD, Nurani LH, Guntarti A. Authentication of citrus peel oils from different species and commercial products using FTIR Spectroscopy combined with chemometrics. Pharmacia. 2024; 71:1-7. Doi:10.3897/pharmacia.71.e118789 DOI: https://doi.org/10.3897/pharmacia.71.e118789
24. Meko OA, Eraga SO, Arhewoh MI. Effect of extraction parameters on some properties of keratin obtained from waste chicken feathers. Trop J Nat Prod Res. 2024; 8(6):7423-7430. Doi:10.26538/tjnpr/v8i6.13 DOI: https://doi.org/10.26538/tjnpr/v8i6.13
25. Omotoso DR, Olubowale VO, Aina FM, Daramola OOO. Phytochemical profiling of Basella alba using gas chromatography-mass spectrometry. Trop J Nat Prod Res. 2024; 8(6):7561-7565. Doi:10.26538/tjnpr/v8i6.36 DOI: https://doi.org/10.26538/tjnpr/v8i6.36
26. Yang Y, Jin H, Zhang J, Wang Y. Determination of total steroid saponins in different species of Paris using FTIR combined with chemometrics. J AOAC Int. 2018; 101(3):732-738. Doi:10.5740/jaoacint.17-0304 DOI: https://doi.org/10.5740/jaoacint.17-0304
27. Joshi R, Kholiya S, Pandey H, Joshi R, Emmanuel O, Tewari A, Kim T, Cho BK. A comparison of ATR-FTIR and Raman spectroscopy for the non-destructive examination of terpenoids in medicinal plants essential oils. Korean J Agric Sci. 2023; 50(4):675-696. Doi:10.7744/kjoas.500408 DOI: https://doi.org/10.7744/kjoas.500408
28. Shlens J. A Tutorial on Principal Component Analysis. arXiv preprint arXiv:14041100. Published online April 3, 2014. Doi:10.48550/arXiv.1404.1100
29. Jolliffe IT and Cadima J. Principal component analysis: a review and recent developments. Philos Trans R Soc A Math Phys Eng Sci. 2016; 374(2065):1-16. Doi:10.1098/rsta.2015.0202
30. Greenacre M, Groenen PJF, Hastie T, D’Enza AI, Markos A, Tuzhilina E. Publisher Correction: Principal component analysis. Nat Rev Methods Primers. 2023; 3(1):22. Doi:10.1038/s43586-023-00209-y DOI: https://doi.org/10.1038/s43586-023-00209-y
31. Rodrigues DP, Mitterer-Daltoé ML, Lima VA de, Barreto-Rodrigues M, Pereira EA. Simultaneous determination of organic acids and sugars in fruit juices by High performance liquid chromatography: characterization and differentiation of commercial juices by principal component analysis. Ciência Rural. 2021; 51(3):1-10. Doi:10.1590/0103-8478cr20200629 DOI: https://doi.org/10.1590/0103-8478cr20200629
32. Ledesma-Escobar CA, Priego-Capote F, Luque de Castro MD. Comparative study of the effect of sample pretreatment and extraction on the determination of flavonoids from lemon (Citrus limon). Abe K, ed. PLoS One. 2016; 11(1):1-16. Doi:10.1371/journal.pone.0148056 DOI: https://doi.org/10.1371/journal.pone.0148056
33. Semenescu AD, Moacă EA, Iftode A, Dehelean CA, Tchiakpe-Antal DS, Vlase L, Vlase AM, Muntean D, Chioibaş R. Phytochemical and nutraceutical screening of ethanol and ethyl acetate phases of Romanian Galium verum herba (Rubiaceae). Molecules. 2023; 28(23):1-29. Doi:10.3390/molecules28237804 DOI: https://doi.org/10.3390/molecules28237804
34. Boukroufa M, Boutekedjiret C, Chemat F. Development of a green procedure of citrus fruits waste processing to recover carotenoids. Resour Technol. 2017; 3(3):252-262. Doi:10.1016/j.reffit.2017.08.007 DOI: https://doi.org/10.1016/j.reffit.2017.08.007
35. Salvo A, Bruno M, La Torre GL, Vadalà R, Mottese AF, Saija E, Mangano V, Casale KE, Cicero N, Dugo G. Interdonato lemon from Nizza di Sicilia (Italy): chemical composition of hexane extract of lemon peel and histochemical investigation. Nat Prod Res. 2016; 30(13):1517-1525. Doi:10.1080/14786419.2015.1115999. DOI: https://doi.org/10.1080/14786419.2015.1115999
36. Kim DS and Lim SB. Extraction of flavanones from immature Citrus unshiu pomace: process optimization and antioxidant evaluation. Sci Rep. 2020; 10(1):19950. Doi:10.1038/s41598-020-76965-8. DOI: https://doi.org/10.1038/s41598-020-76965-8
37. Li BB, Smith B, Hossain M. Extraction of phenolics from citrus peels: II. Enzyme-assisted extraction method. Sep Purif Technol. 2013; 48(2):189-196. Doi:10.1016/j.seppur.2005.07.019 DOI: https://doi.org/10.1016/j.seppur.2005.07.019
38. Teofilović B, Balaž F, Karadžić Banjac M, Grujić-Letić N, Gligorić E, Kovačević S, Podunavac-Kuzmanović S, Stojanović S. Chemometric approach of different extraction conditions on scavenging activity of Helichrisym italicum (Roth) G. Don Extracts. Separations. 2023; 10(8):1-13. Doi:10.3390/separations10080436 DOI: https://doi.org/10.3390/separations10080436
39. Lever J, Krzywinski M, Altman N. Principal component analysis. Nat Methods. 2017; 14(7):641-642. Doi:10.1038/nmeth.4346 DOI: https://doi.org/10.1038/nmeth.4346
40. Rafiq S, Kaul R, Sofi SA, Bashir N, Nazir F, Ahmad Nayik G. Citrus peel as a source of functional ingredient: A review. J Saudi Soc Agric Sci. 2018; 17(4):351-358. Doi:10.1016/j.jssas.2016.07.006. DOI: https://doi.org/10.1016/j.jssas.2016.07.006
41. Brodnjak Vončina D. Chemometrics in analytical chemistry. Nova Biotechnologica et Chimica. 2021; 9(2):211-216. Doi:10.36547/nbc.1280 DOI: https://doi.org/10.36547/nbc.1280
42. Iannucci L. Chemometrics for Data Interpretation: Application of Principal Components Analysis (PCA) to Multivariate Spectroscopic Measurements. IEEE Instrum Meas Mag. 2021; 24(4):42-48. Doi:10.1109/MIM.2021.9448250 DOI: https://doi.org/10.1109/MIM.2021.9448250
43. Lebot V, Labouisse JP, Rivallan R, Kaoh J, Davrieux F. Use of SSR markers and FTIR chemometrics to assess the nobility of Kava cultivars from the Pacific. Food Control. 2024; 164:110598. Doi:10.1016/j.foodcont.2024.110598 DOI: https://doi.org/10.1016/j.foodcont.2024.110598
44. Muyonga JH, Cole CGB, Duodu KG. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004; 86(3):325-332. Doi:10.1016/j.foodchem.2003.09.038 DOI: https://doi.org/10.1016/j.foodchem.2003.09.038