In Silico Molecular Docking Of Beta-Sitosterol and Astaxanthin into Cyclooxygenase Enzymes
Main Article Content
Abstract
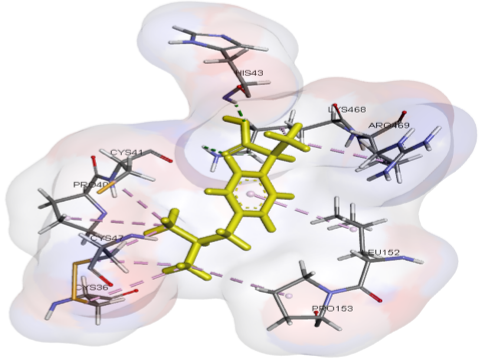
Cyclooxygenase enzymes, mainly COX-1 and COX-2, have been shown to contribute to the regulation of inflammation, pain, and physiological homeostasis, positioning them as critical targets for the development of novel anti-inflammatory therapies. Although NSAIDs have proven their efficacy in COX inhibition, their adverse effects have limited their use, highlighting a research gap that necessitates further exploration of natural compounds, such as beta-sitosterol (BS) and astaxanthin (AXT), as potentially safer, selective COX modulators. This study aimed to explore the potential synergistic effects of BS-AXT combination in modulating COX enzymes through molecular docking to discover their combined activity as safer, natural anti-inflammatory agents. Docking results revealed that the BS-AXT combination significantly enhanced the binding affinity of BS to COX-2, improving from -7.5424 kcal/mol (alone) to -9.2033 kcal/mol in the combination form, indicating a potential synergistic interaction. Consistent with previous docking studies, BS and AXT demonstrated similar binding behaviors, dominated by hydrophobic interactions, particularly with key nonpolar residues within the COX-2 active site. In conclusion, the docking results suggest that the BS-AXT combination could be a natural COX-2 selective inhibitor, supporting the need for further studies to confirm its effectiveness and safety.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Oyesola OO, Tait ED. Prostaglandin regulation of type 2 inflammation: From basic biology to therapeutic interventions. Eur J Immunol. 2021; 51(10):2399–2416. DOI: https://doi.org/10.1002/eji.202048909
2. Faki Y, Er A. Different chemical structures and physiological/pathological roles of cyclooxygenases. Rambam Maimonides Med J. 2021; 12(1):1–13. DOI: https://doi.org/10.5041/RMMJ.10426
3. Araújo PHF, Ramos RS, da Cruz JN, Silva SG, Ferreira EFB, de Lima LR, Macêdo WJC, Espejo-Román JM, Campos JM, Santos CBR. Identification of potential COX-2 inhibitors for the treatment of inflammatory diseases using molecular modeling approaches. Molecules. 2020; 25(18):1–32. DOI: https://doi.org/10.3390/molecules25184183
4. Chan PC, Liao MT, Hsieh PS. The dualistic effect of COX-2-mediated signaling in obesity and insulin resistance. Int J Mol Sci. 2019; 20(13):1–13. DOI: https://doi.org/10.3390/ijms20133115
5. Er-Rajy M, El-Fadili M, Mujwar S, Imtara H, Al kamaly O, Zuhair Alshawwa S, Nasr FA, Zarougui S, Elhallaoui M. Design of novel anti-cancer agents targeting COX-2 inhibitors based on computational studies. Arab J Chem. 2023; 16(10):1–18. DOI: https://doi.org/10.1016/j.arabjc.2023.105193
6. Darkazally AZ, Alnour A, Homsi S. Troxerutin effect on gastric ulcers induced by ketorolac in rats: Relation with oxidative stress. Heliyon. 2024; 10(19):1–11. DOI: https://doi.org/10.1016/j.heliyon.2024.e38893
7. Zhang K, Yuan G, Werdich AA, Zhao Y. Ibuprofen and diclofenac impair the cardiovascular development of zebrafish (Danio rerio) at low concentrations. Environ Pollut. 2020; 258(2020):1–37. DOI: https://doi.org/10.1016/j.envpol.2019.113613
8. Zheng Y, Zhao J, Chang S, Zhuang Z, Waimei S, Li X, Chen Z, Jing B, Zhang D, Zhao G. β-Sitosterol Alleviates Neuropathic Pain by Affect Microglia Polarization through Inhibiting TLR4/NF-κB Signaling Pathway. J Neuroimmune Pharmacol. 2023; 18(4):690–703. DOI: https://doi.org/10.1007/s11481-023-10091-w
9. Tallapalli PS, Reddy YD, Yaraguppi DA, Matangi SP, Challa RR, Vallamkonda B, Ahmad SF, Al-Mazroua HA, Rudrapal M, Dintakurthi SNBKP, Pasala PK. In Silico and In Vivo Studies of β-Sitosterol Nanoparticles as a Potential Therapy for Isoprenaline-Induced Cognitive Impairment in Myocardial Infarction, Targeting Myeloperoxidase. Pharmaceuticals. 2024; 17(8):1–22. DOI: https://doi.org/10.3390/ph17081093
10. Peng YJ, Lu JW, Liu FC, Lee CH, Lee HS, Ho YJ, Hsieh TH, Wu CC, Wang CC. Astaxanthin attenuates joint inflammation induced by monosodium urate crystals. FASEB J. 2020; 34(8):11215–11226. DOI: https://doi.org/10.1096/fj.202000558RR
11. Alam A, Tamkeen N, Imam N, Farooqui A, Ahmed MM, Tazyeen S, Ali S, Malik MZ, Ishrat R. Pharmacokinetic and molecular docking studies of plant-derived natural
compounds to exploring potential anti-Alzheimer activity. In: In Silico Approach for Sustainable Agriculture. Springer Singapore; 2018; 3(2018):217–238.
12. Ijoma IK, Okafor CE, Ajiwe VIE. Computational Studies of 5-methoxypsolaren as Potential Deoxyhemoglobin S Polymerization Inhibitor. Trop J Nat Prod Res. 2024; 8(10):8835–8841. DOI: https://doi.org/10.26538/tjnpr/v8i10.28
13. Zubair MS, Yuyun Y, Musnina WS, Najib A, Nainu F, Arba M, Paneo DR, Praditapuspa EN, Maulana S. Network Pharmacology and Molecular Docking Studies of Ethnopharmacological Plants from Sulawesi as Antidiabetics. Trop J Nat Prod Res. 2025; 9(3):1123–1135. DOI: https://doi.org/10.26538/tjnpr/v9i3.30
14. Baroroh, S.Si., M.Biotek. U, Muscifa ZS, Destiarani W, Rohmatullah FG, Yusuf M. Molecular interaction analysis and visualization of protein-ligand docking using Biovia Discovery Studio Visualizer. Indo J Comput Biol (IJCB). 2023; 2(1):22-30. DOI: https://doi.org/10.24198/ijcb.v2i1.46322
15. Paat VI, Aloanis AA, Maya J, Najoan J. Molecular Docking Of Cyclosenegalin A As Anticancer. Fuller Chem J. 2025; 10(1):26–33.
16. Li X, Xin Y, Mo Y, Marozik P, He T, Guo H. The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Molecules. 2022; 27(523):1–15. DOI: https://doi.org/10.3390/molecules27020523
17. Tuj Johra F, Kumar Bepari A, Tabassum Bristy A, Mahmud Reza H. A mechanistic review of β-carotene, lutein, and zeaxanthin in eye health and disease. Antioxidants. 2020; 9(11):1–21. DOI: https://doi.org/10.3390/antiox9111046
18. Md Idris MH, Mohd Amin SN, Mohd Amin SN, Nyokat N, Khong HY, Selvaraj M, Zakaria ZA, Shaameri Z, Hamzah AS, Teh LK, Salleh MZ. Flavonoids as dual inhibitors of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX): molecular docking and in vitro studies. Beni Suef Univ J Basic Appl Sci. 2022; 11(117):1–9. DOI: https://doi.org/10.1186/s43088-022-00296-y
19. Ahmadi M, Bekeschus S, Weltmann KD, von Woedtke T, Wende K. Non-steroidal anti-inflammatory drugs: recent advances in the use of synthetic COX-2 inhibitors. RSC Med Chem. 2022; 13(5):471–496. DOI: https://doi.org/10.1039/D1MD00280E
20. Musfiroh I, Kartasasmita RE, Ibrahim S, Muchtaridi M, Hidayat S, Ikram NKK. Stability Analysis of the Asiatic Acid-COX-2 Complex Using 100 ns Molecular Dynamic Simulations and Its Selectivity against COX-2 as a Potential Anti-Inflammatory Candidate. Molecules. 2023; 28(9):1–11. DOI: https://doi.org/10.3390/molecules28093762
21. Desantis F, Miotto M, Di Rienzo L, Milanetti E, Ruocco G. Spatial organization of hydrophobic and charged residues affects protein thermal stability and binding affinity. Sci Rep. 2022; 12(1):1–13. DOI: https://doi.org/10.1038/s41598-022-16338-5
22. Al-Mughram MH, Catalano C, Herrington NB, Safo MK, Kellogg GE. 3D interaction homology: The hydrophobic residues alanine, isoleucine, leucine, proline and valine play different structural roles in soluble and membrane proteins. Front Mol Biosci. 2023; 10(1):1–24. DOI: https://doi.org/10.3389/fmolb.2023.1116868
23. Singh CD, Kumar Gupta A, Deepak M, Chaudhary S. Silico and Analytical Evaluation of Beta-Sitosterol From Anogeissus Pendula As A Potential Therapeutic Agent Against Hyperlipidemia. J Neonatal Surg ISSN [Internet]. 2025; 2(1):12–16. Available from: https://www.jneonatalsurg.com
24. Owoloye AJ, Ligali FC, Enejoh OA, Musa AZ, Aina O, Idowu ET, Oyebola KM. Molecular docking, simulation and binding free energy analysis of small molecules as Pf HT1 inhibitors. PLoS One. 2022; 17(8):1–18. DOI: https://doi.org/10.1371/journal.pone.0268269
25. Naznin NE, Mazumder T, Reza MS, Jafrin S, Alshahrani SM, Alqahtani AM, Shahid Ud Daula AFM. Molecular Docking Supported Investigation of Antioxidant, Analgesic and Diuretic Effects of Costus Specious Rhizome. Bull Chem Soc Ethiop. 2022;36(3):627–640. DOI: https://doi.org/10.4314/bcse.v36i3.12
26. Ma LS, Jia XT, Hu FQ, Zheng YJ, Huang XF, Rausch-Fan X, Fan XC. Mechanism of Lycium barbarum in treating periodontitis based on network pharmacology, molecular docking, and experimental validation. Clin Oral Investig. 2025; 29(219):1–14. DOI: https://doi.org/10.1007/s00784-025-06313-5
27. Muzyamba S, Singh I Sen. GC-MS, GNPS and METLIN Assisted Phytochemical Profiling, Bioactivity Study and Molecular Docking Analysis of Paropsia brazzeana Root Bark, a Medicinal Plant in Zambia. Sys Rev Pharm [Internet]. 2024; 15(1):1–14. Available from: https://www.researchgate.net/publication/378549828
28. Adelia M, Setiawansyah A, Sitindaon RS. Potential activity of Stevia rebaudiana Bert. in Inhibiting Cyclooxygenase and Lipooxygenase Enzymes as Anti-inflammatory Candidates: A Molecular Docking Study and ADMET Prediction. J Drug Deliv Ther. 2023; 13(7):75–86. DOI: https://doi.org/10.22270/jddt.v13i7.6137
29. Oladoja FA, Awodele O, Oreagba IA, Irokosu ES, Oyinloye EO, Murtala AA. Anti-inflammatory activity and molecular docking studies of the hydromethanolic leaf extract of Baphia longipedicellata brumitt in rats. Pharmacol Res - Mod Chin Med. 2024; 13(2024):1–9. DOI: https://doi.org/10.1016/j.prmcm.2024.100512
30. Jain S, Ganeshpurkar A, Dubey N. Molecular Docking of some Neem Constituents with COX-2 and NOs: An in silico Study. Pharmacogn Commun. 2020; 10(3):134–135. DOI: https://doi.org/10.5530/pc.2020.3.26
31. Utami W, Aziz HA, Nasrudin D, Kusmawan A, Anwar Z, Maulana M, Daryanto M. Investigating the potency of bioactive compounds from Ficus religiosa as anti-inflammatory agent. J Phys Conf Ser. 2021; 1869(2021):1–6. DOI: https://doi.org/10.1088/1742-6596/1869/1/012022
32. Akinloye OA, Akinloye DI, Onigbinde SB, Metibemu DS. Phytosterols demonstrate selective inhibition of COX-2: In-vivo and in-silico studies of Nicotiana tabacum. Bioorg Chem. 2020; 102(2020):1–13. DOI: https://doi.org/10.1016/j.bioorg.2020.104037
33. Zhang G, Qi X, He L, Wang X, Zhao Y, Wang Q, Han J, Wang Z, Ding Z, Liu M. Non-covalent complexes of lutein/zeaxanthin and whey protein isolate formed at
different pH levels: Binding interactions, storage stabilities, and bioaccessibilities. Curr Res Food Sci. 2024; 8(2024):1–10. DOI: https://doi.org/10.1016/j.crfs.2024.100778
34. Shi G, Gu L, Jung H, Chung WJ, Koo S. Apocarotenals of Phenolic Carotenoids for Superior Antioxidant Activities. ACS Omega. 2021; 6(38):25096–25108. DOI: https://doi.org/10.1021/acsomega.1c04432
35. Jafari Z, Bigham A, Sadeghi S, Dehdashti SM, Rabiee N, Abedivash A, Bagherzadeh M, Nasseri B, Karimi-Maleh H, Sharifi E, Varma RS, Makvandi P. Nanotechnology-Abetted Astaxanthin Formulations in Multimodel Therapeutic and Biomedical Applications. J Med Chem. 2022; 65(1):2–36. DOI: https://doi.org/10.1021/acs.jmedchem.1c01144
36. Sadoud M, Zidane A, Metlef S, Nemar F, Riazi A. The properties of astaxanthin extracted from Algerian green microalga Haematococcus pluvialis. Afr J Biol Sci [Internet]. 2024; 6(15):9583–9599. Available from: https://doi.org/10.48047/AFJBS.6.15.2024.9582-9599
37. Akinade TC, Babatunde OO, Adedara AO, Adeyemi OE, Otenaike TA, Ashaolu OP, Johnson TA, Terriente-Felix A, Whitworth AJ, Abolaji AO. Protective capacity of
carotenoid trans-astaxanthin in rotenone-induced toxicity in Drosophila melanogaster. Sci Rep. 2022; 12(1):1–13. DOI: https://doi.org/10.1038/s41598-022-08409-4
38. Liu Z, Li Y, Bao J, Li S, Wen Y, Zhang P, Feng J, Wang Y, Tian L, Jie Y. Astaxanthin ameliorates benzalkonium chloride–induced dry eye disease through suppressing inflammation and oxidative stress via Keap1-Nrf2/HO-1 signaling pathways. Animal Model Exp Med. 2025; 6;1–24. DOI: https://doi.org/10.1002/ame2.70000
39. Yang S, Lin X, Shi B, Meng J, Liu L, Yan L, Xie F. Hydrastinine and β-sitosterol Synergistically Target Acute Myelocytic Leukemia in a Ferroptosis-Related Prognostic Model. Res Sq [Internet]. 2023; 1–25. Available from: https://www.researchsquare.com/article/rs-3770830/v1 DOI: https://doi.org/10.21203/rs.3.rs-3770830/v1
40. Shinde P, Singh G, Kardile D, Salunke M, Kashid S. Beta-Sitosterol and Stigmasterol as Synergestic Anticancer Potential in Breast Cancer Isolated from Euphorbiaceae Family Plants. Afr J Biomed Res [Internet]. 2024; 27(4):1929–1934. Available from: https://africanjournalofbiomedicalresearch.com/index.php/AJBR/article/view/3966 DOI: https://doi.org/10.53555/AJBR.v27i4S.3966
41. Cai L, Cai Y, Yu R, Song A, Yang C, Yang W, Jiang F. Synergistic Analgesic Effects of Astaxanthin Combined
with Celecoxib on a Mouse 2 Bone Cancer Pain Model: From Behavioral Validation to Target Prediction. SSRN [Internet]. 2025; 1–48. Available from: https://ssrn.com/abstract=5243617
42. Marzagalli M, Battaglia S, Raimondi M, Fontana F, Cozzi M, Ranieri FR, Sacchi R, Curti V, Limonta P. Anti-
Inflammatory and Antioxidant Properties of a New Mixture of Vitamin C, Collagen Peptides, Resveratrol, and Astaxanthin in Tenocytes: Molecular Basis for Future Applications in Tendinopathies. Mediators Inflamm. 2024; 5273198 1–17. DOI: https://doi.org/10.1155/2024/5273198