The Stimulatory Effect of Porcine Placenta Extract on in Vitro Biological Activities of Keratinocytes and Fibroblasts
Main Article Content
Abstract
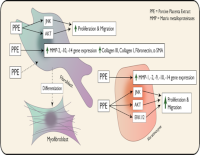
Porcine placenta extract (PPE), a synergistic bioactive compound, enhances cellular functions such as proliferation, migration, and cellular signaling. Keratinocytes and fibroblasts play essential roles during wound healing, particularly in tissue regeneration. However, the direct impact of PPE on biological activities of keratinocytes and fibroblasts remains underexplored. This study aimed to elucidate the stimulatory impact of PPE on the proliferation, migration, and gene expression of matrix metalloproteinases (MMPs) and extracellular matrix (ECM)-related proteins in human keratinocytes and fibroblasts. Human keratinocyte (HaCaT) and fibroblast cell lines (Hs 895.Sk) were treated with specific concentrations of PPE (2.5, 5, 10, 12.5, 15, and 50 µg/mL). Cell proliferation and migration were assessed using MTT and scratch assays, respectively. Quantitative real-time PCR was performed to evaluate the expression of MMPs and ECM-related genes. Western blot was employed to investigate the activation of ERK1/2, AKT, JNK signaling pathways, and cyclin D1 expression. PPE significantly promoted the keratinocyte/fibroblast proliferation and migration in a concentration-dependent manner via activation of ERK1/2, AKT, and JNK signaling pathway. PPE also markedly upregulated the expression of MMP-2, MMP-10, and MMP-14 both in keratinocytes and fibroblasts, as well as α-SMA, fibronectin, collagen I, and collagen III in fibroblasts. Porcine placenta extract (PPE) enhances keratinocyte and fibroblast proliferation/migration by activating JNK, ERK1/2, and PI3K/AKT signaling pathways. PPE also upregulates MMP- and ECM-related gene expression including myofibroblast differentiation.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Amiri N, Golin AP, Jalili RB, Ghahary A. Roles of cutaneous cell-cell communication in wound healing outcome: An emphasis on keratinocyte-fibroblast crosstalk. Exp Dermatol. 2022;31(4):475-484. DOI: https://doi.org/10.1111/exd.14516
Diller RB, Tabor AJ. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics. 2022;7(3):87. DOI: https://doi.org/10.3390/biomimetics7030087
Tefft JB, Chen CS, Eyckmans J. Reconstituting the dynamics of endothelial cells and fibroblasts in wound closure. APL Bioengineering. 2021;5(1). DOI: https://doi.org/10.1063/5.0028651
Kandhwal M, Behl T, Singh S, Sharma N, Arora S, Bhatia S, Al-Harrasi A, Sachdeva M, Bungau S. Role of matrix metalloproteinase in wound healing. Am J Transl Res. 2022;14(7):4391-4405.
Protzman NM, Mao Y, Long D, Sivalenka R, Gosiewska A, Hariri RJ, Brigido SA. Placental-Derived Biomaterials and Their Application to Wound Healing: A Review. Bioengineering (Basel). 2023;10(7). DOI: https://doi.org/10.3390/bioengineering10070829
Wu CH, Chang GY, Chang WC, Hsu CT, Chen RS. Wound healing effects of porcine placental extracts on rats with thermal injury. Br J Dermatol. 2003;148(2):236-245. DOI: https://doi.org/10.1046/j.1365-2133.2003.05164.x
Failla CM, Odorisio T, Cianfarani F, Schietroma C, Puddu P, Zambruno G. Placenta growth factor is induced in human keratinocytes during wound healing. J Invest Dermatol. 2000;115(3):388-395. DOI: https://doi.org/10.1046/j.1523-1747.2000.00085.x
Hong JW, Lee WJ, Hahn SB, Kim BJ, Lew DH. The effect of human placenta extract in a wound healing model. Ann Plast Surg. 2010;65(1):96-100. DOI: https://doi.org/10.1097/SAP.0b013e3181b0bb67
Worawat Songjang, Chatchai Nensat, Rutaiwan Tohtong, Tuangporn Suthiphongchai, Suchada Phimsen, Panthip Rattanasinganchan, Pornphimon Metheenukul, Waraporn Kasekarn, Arunya Jiraviriyakul. Porcine placenta extract induced Akt, ERK, and JNK signaling to heighten the osteogenic activity of human osteoblasts. J Appl Pharm Sci. 2022;Volume: 12:018-025. DOI: https://doi.org/10.7324/JAPS.2022.120803
Padhomchai Pumbthongthae SR, Tavan Janvilisri, Puey Ounjai, Analysis of Protein Profile of Crude porcine placenta extract. The 50th National Graduate Research Conference; 2020; King Mongkut's Institute of Technology Ladkrabang, Bangkok.
Pfleger T, Ortmayr K, Steiner K, Zaher R, Seiser S, Elbe-Bürger A, Heiss E, Klang V. Radical scavenging effect of skin delivery systems using Korean red ginseng extract and assessment of their biocompatibility with human primary dermal fibroblasts and HaCaT keratinocytes. Int J Pharm. 2025;674:125477. DOI: https://doi.org/10.1016/j.ijpharm.2025.125477
Jiang L, Yi Q, Sun Z, Lin Y. ZNT1 Regulates the Proliferation, Migration and Invasion of HaCaT Cells Infected with HPV Through the PI3K/Akt Pathway. Indian J Dermatol. 2024;69(2):201. DOI: https://doi.org/10.4103/ijd.ijd_775_23
Nensat C, Songjang W, Tohtong R, Suthiphongchai T, Phimsen S, Rattanasinganchan P, Metheenukul P, Kumphune S, Jiraviriyakul A. Porcine placenta extract improves high-glucose-induced angiogenesis impairment. BMC Complement Med Ther. 2021;21(1):66. DOI: https://doi.org/10.1186/s12906-021-03243-z
Huang L, Chin L-C, Kimura K, Nakahata Y. Human Placental Extract Delays In Vitro Cellular Senescence through the Activation of NRF2-Mediated Antioxidant Pathway. Antioxidants. 2022;11(8):1545. DOI: https://doi.org/10.3390/antiox11081545
Tansathien K, Ngawhirunpat T, Rangsimawong W, Patrojanasophon P, Opanasopit P, Nuntharatanapong N. In Vitro Biological Activity and In Vivo Human Study of Porcine-Placenta-Extract-Loaded Nanovesicle Formulations for Skin and Hair Rejuvenation. Pharmaceutics. 2022;14(9):1846. DOI: https://doi.org/10.3390/pharmaceutics14091846
Nikoloudaki G, Brooks S, Peidl AP, Tinney D, Hamilton DW. JNK Signaling as a Key Modulator of Soft Connective Tissue Physiology, Pathology, and Healing. Int J Mol Sci. 2020;21(3):1015. DOI: https://doi.org/10.3390/ijms21031015
Zhao B, Liu JQ, Zheng Z, Zhang J, Wang SY, Han SC, Zhou Q, Guan H, Li C, Su LL, Hu DH. Human amniotic epithelial stem cells promote wound healing by facilitating migration and proliferation of keratinocytes via ERK, JNK and AKT signaling pathways. Cell Tissue Res. 2016;365(1):85-99. DOI: https://doi.org/10.1007/s00441-016-2366-1
Hammouda MB, Ford AE, Liu Y, Zhang JY. The JNK Signaling Pathway in Inflammatory Skin Disorders and Cancer. Cells. 2020;9(4):857. DOI: https://doi.org/10.3390/cells9040857
Olschläger V, Schrader A, Hockertz S. Comparison of primary human fibroblasts and keratinocytes with immortalized cell lines regarding their sensitivity to sodium dodecyl sulfate in a neutral red uptake cytotoxicity assay. Arzneimittelforschung. 2009;59(3):146-152. DOI: https://doi.org/10.1055/s-0031-1296378