The Antioxidant Activity of Muntingia calabura L. Leaf Extract and its Effect in Acute Gout Arthritis Rat Model: Reactive Oxygen Species Modulation
Main Article Content
Abstract
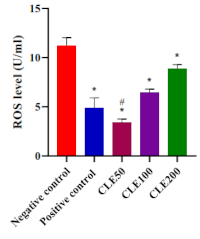
Gout arthritis is an inflammatory joint condition caused by uric acid crystal accumulation. The inflammatory mechanism involves complex pathways that increase reactive oxygen species (ROS) levels. Muntingia calabura L. leaves contain bioactive compounds, such as phenolics, including flavonoids, which are known to reduce ROS production by mitigating pro-inflammatory signaling pathways. This study aimed to evaluate the phenolic content and effectiveness of Muntingia calabura L. leaves extract as an anti-inflammation and antioxidant agent in regulating ROS levels in Wistar rats with acute gout arthritis. Five groups of rats were used, which consisted of positive control, negative control, and treatment groups receiving the extract at doses of 50, 100, and 200 mg/kg BW. Folin-Ciocalteu assay revealed a high phenolic content of 26.12 ± 0.18%, with strong antioxidant activity (IC50 = 23.85 ± 0.16 µg/mL). In line with this, LC-MS-MS showed that flavonoids were the most predominant compounds in the extract. Meanwhile, the extract administration slightly reduced pain-induced monosodium urate (MSU) crystal injection. Interestingly, 50 mg/kg BW of the extract was identified as the most effective dose in reducing MSU-induced ROS production—even stronger than that of colchicine (p = 0.0049). This study highlighted the significant antioxidant activity of the extract due to its phenolic content, which markedly lowered ROS levels in the animal model.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Nguyen LK, Tran CV, Pham ND, Tran TV. Phytochemical screening, antioxidant and xanthine oxidase inhibitory activities of Vitis Heyneana Schult. Trop J Nat Prod Res. 2023;7(9):3981-3988. http://www.doi.org/10.26538/tjnpr/v7i9.20 DOI: https://doi.org/10.26538/tjnpr/v7i9.20
2.Singh JA, Gaffo A. Gout epidemiology and comorbidities. Semin Arthritis Rheum. 2020;50(3):S11–S16. https://doi.org/10.1016/j.semarthrit.2020.04.008 DOI: https://doi.org/10.1016/j.semarthrit.2020.04.008
3.Kim SK. The mechanism of the NLRP3 inflammasome activation and pathogenic implication in the pathogenesis of gout. J Rheum Dis. 2022;29(3):140–153. https://doi.org/10.4078/jrd.2022.29.3.140 DOI: https://doi.org/10.4078/jrd.2022.29.3.140
4.Alkadi H, Khubeiz MJ. Colchicine: A Review About Chemical Structure and Clinical Using. Infect Disord Drug Targets. 2017;17(July). https://doi.org/ 10.2174/1871526517666171017114901
5.Stewart S, Yang KCK, Atkins K, Dalbeth N, Robinson PC. Adverse events during oral colchicine use: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2020;22(1). https://doi.org/10.1186/s13075-020-2120-7 DOI: https://doi.org/10.1186/s13075-020-2120-7
6.Al-Khayri JM, Sahana GR, Nagella P, Joseph B V, Alessa FM, Al-Mssallem MQ. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Mol. 2022;27(9):2901. https://doi.org/10.3390/molecules27092901 DOI: https://doi.org/10.3390/molecules27092901
7.Vuolo MM, Lima VS, Junior MRM. Phenolic compounds: Structure, classification, and antioxidant power. In: Bioactive compounds. Elsevier; 2019. p. 33–50. https://doi.org/10.1016/B978-0-12-814774-0.00002-5 DOI: https://doi.org/10.1016/B978-0-12-814774-0.00002-5
8.Kumar N, Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol Rep. 2019;24:1–10. https://doi.org/10.1016/j.btre.2019.e00370 DOI: https://doi.org/10.1016/j.btre.2019.e00370
9.RosyidulIbad M, Nasution TH, Andarini S. Effect of kersen leaf extract (Muntingia calabura) on the erythema degree in inflammatory process of guinea pigs (Cavia porcellus) with shallow second degree burns. J Nurs Sci. 2013;1(2):157–161.
10.Leyva-López N, Gutierrez-Grijalva EP, Ambriz-Perez DL, Heredia JB. Flavonoids as cytokine modulators: a possible therapy for inflammation-related diseases. Int J Mol Sci. 2016;17(6):921. https://doi.org/10.3390/ijms17060921 DOI: https://doi.org/10.3390/ijms17060921
11.Choy KW, Murugan D, Leong X fang, Abas R, Alias A, Mustafa MR. Flavonoids as Natural Anti-Inflammatory Agents Targeting Nuclear Factor-Kappa B (NFκB) Signaling in Cardiovascular Diseases: A Mini Review. Front Pharmacol. 2019;10:1–8 DOI: https://doi.org/10.3389/fphar.2019.01295
12.Syabania M, Pambudi DB, Wirasti W, Rahmatullah S. Characterization and Evaluation of Kersen Leaf Extract (Muntingia calabura L.) Granule by Wet Granulation Method. In: Proceedings of the National Seminar on Health. 2021. p. 1737–1746. https://doi.org/10.3389/fphar.2019.01295 DOI: https://doi.org/10.48144/prosiding.v1i.926
13.Vonna A, Desiyana LS, Hafsyari R, Illian DN. Phytochemical Analysis and Characterization of Ethanol Extract of Kersen Leaf (Muntingia calabura L.). J Bioleuser. 2021;5(1).
14.Ghosal P, Chandra S, Choudhary AN, Saklani S. Sonchus arvensis: Antioxidant activity, phenolic profile, and phytochemical screening. Kariri Sci - Cecape Biol Health. 2023;1(1). https://doi.org/10.29327/2256856.2023.1-8 DOI: https://doi.org/10.29327/2256856.2023.1-8
15.Farida Y, Qodriah R, Nilesh S. Quality parameters and determination of total flavonoid levels from the highest antioxidant activity of ethanol 70% extract jackfruit peel (Artocarpus Heterophyllus L.) by maceration, reflux, and ultrasonic methods. Int J App Pharm. 2022;100–103. https://dx.doi.org/10.22159/ijap.2022.v14s3 DOI: https://doi.org/10.22159/ijap.2022.v14s3.21
16.Elhady SS, Abdelhameed RFA, Mehanna ET, Wahba AS, Elfaky MA, Koshak AE, Noor AO, Bogari HA, Malatani, RT, Goda MS. Metabolic profiling, chemical composition, antioxidant capacity, and in vivo hepato-and nephroprotective effects of Sonchus cornutus in mice exposed to cisplatin. Antioxidants. 2022;11(5):819. doi: 10.3390/antiox11050819 DOI: https://doi.org/10.3390/antiox11050819
17.Saputra FI. Immunomudulatory effect of kersen (Muntingia calabura L.) leaf extract in mice with carbon clearance method [thesis]. Padang: Universitas Andalas; 2021
18.Parisa N, Hidayat R, Maritska Z, Prananjaya BA. Evaluation of the anti-gout effect of Sonchus Arvensis on monosodium urate crystal-induced gout arthritis via anti-inflammatory action - an in vivo study. Med Pharm Rep. 2021;94(3):358–365. https://doi.org/10.15386/mpr-1959 DOI: https://doi.org/10.15386/mpr-1959
19.Parisa N, Kamaluddin MT, Saleh MI, Sinaga E, Partan RU, Irfannuddin, Mangunsong S. The Effectiveness of Tempuyung Leaves’ Water Fraction for Inflammation Prevention of Wistar Rats in an Acute Gout Arthritis Model. Indonesian J Pharm. 2025;0(0). https://jurnal.ugm.ac.id/v3/IJP/article/view/14504
20.Mergy MA, Gowrishankar R, Davis GL, Jessen TN, Wright J, Stanwood GD, Hahn MK, Blakely, RD. Genetic targeting of the amphetamine and methylphenidate-sensitive dopamine transporter: on the path to an animal model of attention-deficit hyperactivity disorder. Neurochem Int. 2014;73:56–70. http://dx.doi.org/10.1016/j.neuint.2013.11.009 DOI: https://doi.org/10.1016/j.neuint.2013.11.009
21.Prayitno SA, Rahim AR. Comparison of Extracts (Ethanol And Aquos Solvents) Muntingia calabura Leaves on Total Phenol, Flavonid And Antioxidant (Ic50) Properties. Kontribusia: R D C D. 2020;3(2):319–325. http://dx.doi.org/10.30587/kontribusia.v3i2.1451 DOI: https://doi.org/10.30587/kontribusia.v3i2.1451
22.Upadhye M, Kuchekar M, Pujari R, Kadam S, Gunjal P. Muntingia calabura: A comprehensive review. J Pharm Biol Sci. 2021;9(2):81–87. https://doi.org/ 10.18231/j.jpbs.2021.011 DOI: https://doi.org/10.18231/j.jpbs.2021.011
23.Sinaga SP, Lumbangaol DA, Iksen RFR, Gurning K. Determination of phenolic, flavonoid content, antioxidant and antibacterial activities of seri (Muntingia calabura L.) leaves ethanol extract from North Sumatera, Indonesia. Rasayan J Chem. 2022;15(02):1534–1538. http://doi.org/10.31788/RJC.2022.1526730 DOI: https://doi.org/10.31788/RJC.2022.1526730
24.Akomeng N, Adusei S. Organic solvent extraction and spectrophotometric quantification of total phenolic content of soil. Heliyon. 2021;7(11). https://doi.org/10.1016/j.heliyon.2021.e08388 DOI: https://doi.org/10.1016/j.heliyon.2021.e08388
25.Pant P, Pandey S, Dall’Acqua S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem Biodivers. 2021;18(11):e2100345. https://doi.org/10.1002/cbdv.202100345 DOI: https://doi.org/10.1002/cbdv.202100345
26.Tirado-Kulieva VA, Hernández-Martínez E, Choque-Rivera TJ. Phenolic compounds versus SARS-CoV-2: An update on
the main findings against COVID-19. Heliyon. 2022;8(9). https://doi.org/10.1016/j.heliyon.2022.e10702 DOI: https://doi.org/10.1016/j.heliyon.2022.e10702
27.Sun W, Shahrajabian MH. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Mol. 2023;28(4):1845. https://doi.org/10.3390/molecules28041845 DOI: https://doi.org/10.3390/molecules28041845
28.Zakaria ZA. Free radical scavenging activity of some plants available in Malaysia. Iran J Pharmacol Ther. 2007;6:87–91. http://ijpt.iums.ac.ir/article-1-106-en.html
29.Mahmood ND, Nasir NLM, Rofiee MS, Tohid SFM, Ching SM, Teh LK, Salleh MZ, Zakaria ZA. Muntingia calabura: A review of its traditional uses, chemical properties, and pharmacological observations. Pharm Biol. 2014;52(12):1598–1623. https://doi.org/10.3109/13880209.2014.908397 DOI: https://doi.org/10.3109/13880209.2014.908397
30.Jisha N, Vysakh A, Vijeesh V, Latha MS. Anti-inflammatory efficacy of methanolic extract of Muntingia calabura L. leaves in Carrageenan induced paw edema model. Pathophysiology. 2019;26(3–4):323–330. https://doi.org/10.1016/j.pathophys.2019.08.002 DOI: https://doi.org/10.1016/j.pathophys.2019.08.002
31.Balan T, Sani MHM, Ahmad SHM, Suppaiah V, Mohtarrudin N, Zakaria ZA. Antioxidant and anti-inflammatory activities contribute to the prophylactic effect of semi-purified fractions obtained from the crude methanol extract of Muntingia calabura leaves against gastric ulceration in rats. J Ethnopharmacol. 2015;164:1–15. https://doi.org/10.1016/j.jep.2014.12.017 DOI: https://doi.org/10.1016/j.jep.2014.12.017
32.Hnamte S, Parasuraman P, Ranganathan S, Ampasala DR, Reddy D, Kumavath RN, Suchiang K, Mohanty SW, Busi S. Mosloflavone attenuates the quorum sensing controlled virulence phenotypes and biofilm formation in Pseudomonas aeruginosa PAO1: In vitro, in vivo and in silico approach. Microb Pathog. 2019;131:128–134. https://doi.org/10.1016/j.micpath.2019.04.005 DOI: https://doi.org/10.1016/j.micpath.2019.04.005
33.Hájek J. Biological activity of antioxidant compounds in monocytes THP-1 [thesis]. Prague: Charles University; 2015
34.Kim SY, Hassan AH, Chung KS, Kim SY, Han HS, Lee HH, Jung SH, Lee KY, Shin JS, Jang E, Yoon S. Mosloflavone-resveratrol hybrid TMS-HDMF-5z exhibits potent in vitro and in vivo anti-inflammatory effects through NF-κB, AP-1, and JAK/STAT inactivation. Front Pharmacol. 2022;13:857789. https://doi.org/10.3389/fphar.2022.857789 DOI: https://doi.org/10.3389/fphar.2022.857789
35.Amarowicz R, Pegg RB. Protection of natural antioxidants against low-density lipoprotein oxidation. Adv Food Nutr Res. 2020;93:251-291. https://doi.org/10.1016/bs.afnr.2020.04.002 DOI: https://doi.org/10.1016/bs.afnr.2020.04.002
36.Navarrete A, Balderas-López JL, Rosas-Canales JG, Tapia-Álvarez GR, Alfaro-Romero A, Aviles-Rosas VH, Rodríguez-Ramos F, Avula B, Khan IA. Flavones isolated from Pseudognaphalium liebmannii with tracheal smooth muscle relaxant properties. Nat Prod Res. 2025;39(6):1461–1466. https://doi.org/10.1080/14786419.2023.2300402 DOI: https://doi.org/10.1080/14786419.2023.2300402
37.Aloud AA, Veeramani C, Govindasamy C, Alsaif MA, El Newehy AS, Al-Numair KS. Galangin, a dietary flavonoid, improves antioxidant status and reduces hyperglycemia-mediated oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2017;22(6):290–300. https://doi.org/10.1080/13510002.2016.1273437 DOI: https://doi.org/10.1080/13510002.2016.1273437
38.Lin K, Fu D, Wang Z, Zhang X, Zhu C. Analgesic and anti-inflammatory effects of galangin: A potential pathway to inhibit transient receptor potential vanilloid 1 receptor activation. Korean J Pain. 2024;37(2):151–163. https://doi.org/10.3344/kjp.23363 DOI: https://doi.org/10.3344/kjp.23363
39.Wang Y, Zhang Y, Yu Z, Bai Y, Zhu M, Lei Y, Dong B, Zhang Q, Gu Q, Xiang Jian. Developing Monosodium Urate Monohydrate Crystals–Induced Gout Model in Rodents and Rabbits. Curr Protoc. 2025;5(3):e70114. https://doi.org/10.1002/cpz1.70114 DOI: https://doi.org/10.1002/cpz1.70114
40.Zhang G, Lin Y, Chen X, Qin J, He Y, Liu T, Zhang L, Zhang L. Cinnamomi cortex extract mitigated monosodium urate-induced acute gouty arthritis in rats through nuclear factor-κB-NOD-like receptor thermal protein domain associated protein 3 signaling pathway. J Vet Med Sci. 2024;86(6):623–630. https://doi.org/10.1292/jvms.23-0085 DOI: https://doi.org/10.1292/jvms.23-0085
41.Dai X, Fang X, Xia Y, Li M, Li X, Wang Y, Tao J, Li X. ATP-activated P2X7R promote the attack of acute gouty arthritis in rats through activating NLRP3 inflammasome and inflammatory cytokine production. J Inflamm Res. 2022;1237–1248. https://doi.org/10.2147/JIR.S351660. eCollection 2022 DOI: https://doi.org/10.2147/JIR.S351660
42.Lee YM, Cho SN, Son E, Song CH, Kim DS. Apamin from bee venom suppresses inflammation in a murine model of gouty arthritis. J Ethnopharmacol. 2020;257:112860. https://doi.org/10.1016/j.jep.2020.112860 DOI: https://doi.org/10.1016/j.jep.2020.112860
43.Plotz B, Pillinger M, Samuels J. Colchicine and clinical trials for hand osteoarthritis. Osteoarthr Cartil. 2022;30(1):172-173. https://doi.org/10.1016/j.joca.2020.12.026 1063-4584 DOI: https://doi.org/10.1016/j.joca.2020.12.026
44.Latourte A, Pascart T, Flipo RM, Chalès G, Coblentz-Baumann L, Cohen-Solal A, Ea HK, Grichy J, Letavernier E, Lioté F, Ottaviani S, Sigwalt P, Vandecandelaere G, Richette P, Bardin T. 2020 Recommendations from the French Society of Rheumatology for the management of gout: Management of acute flares. J Bone Spine. 2020;87(5):387–393. https://doi.org/10.1016/j.jbspin.2020.05.001 DOI: https://doi.org/10.1016/j.jbspin.2020.05.001
45.Cheng JJ, Ma XD, Ai GX, Yu QX, Chen XY, Yan F, Li YC, Xie JH, Su ZR, Xie QF. Palmatine protects against MSU-induced gouty arthritis via regulating the NF-κB/NLRP3 and Nrf2 pathways. Drug Des Devel Ther. 2023;2119–2132. https://doi.org/10.2147/DDDT.S356307 DOI: https://doi.org/10.2147/DDDT.S356307
46.Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt Jr PIH. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J. 2016;473(24):4527–4550. https://doi.org/10.1042/BCJ20160503C DOI: https://doi.org/10.1042/BCJ20160503C
47.Zălar DM, Pop C, Buzdugan E, Kiss B, Ştefan MG, Ghibu S, Crişan D, Buruiană-Simic A, Grozav A, Borda IM, Mogoșan CI. Effects of colchicine in a rat model of diet-induced hyperlipidemia. Antioxidants. 2022;11(2):230. https://doi.org/10.3390/antiox11020230 DOI: https://doi.org/10.3390/antiox11020230
48.El Hasbani G, Jawad A, Uthman I. Colchicine: An ancient drug with multiple benefits. Curr Pharm Des. 2021;27(26):2917-2924. https://doi.org/10.2174/1381612826666201023144320 DOI: https://doi.org/10.2174/1381612826666201023144320
49.Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299. https://doi.org/10.1016/j.foodchem.2019.125124 DOI: https://doi.org/10.1016/j.foodchem.2019.125124