Protective Effects of Glycine max (L.) Merr. Ethanol Extract against Acetaminophen-Induced Nephrotoxicity and Hepatotoxicity in Wistar Rats
Main Article Content
Abstract
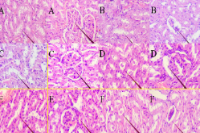
Acetaminophen, widely used as an analgesic and antipyretic, poses risks of nephrotoxicity and hepatotoxicity on prolonged use, leading to cumulative organ damage. Glycine max (edamame), recognized for its antioxidant, and anti-inflammatory properties, can offer potential protective effects against this form of chronic toxicity. This study aimed to evaluate the hepatorenal protective effect of edamame ethanol extract in acetaminophen-induced organ damage in rats. Wistar rats were divided into six groups: normal control, negative control given acetaminophen (1 g/kg) only, positive control given vitamin C (28 mg/kg) and acetaminophen, and three intervention groups receiving edamame extract at 200, 400, and 600 mg/kg once daily for ten days, acetaminophen was administered on the eleventh day. Hepatorenal protective effects of the extract was evaluated by measuring serum levels of liver and kidney function parameters, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea, and creatinine. Effect of the extract on inflammatory biomarkers [kidney injury molecule-1 (KIM-1) and tumor necrosis factor- (TNF-)] as well as kidney and liver histopathology were also assessed. Results showed significant reductions in serum levels of urea (32%) and creatinine (28%) in the higher-dose groups, with marked improvements in kidney and liver histopathology, particularly in tubular and glomerular structures. ALT and AST levels were also significantly decreased by up to 35%, underscoring the extract's hepatoprotective potential. Although reductions in KIM-1 and TNF-α levels were not significant, histological assessments indicated decreased inflammation and oxidative damage. This study highlights edamame ethanol extract as a promising natural agent for mitigating cumulative acetaminophen-induced nephrotoxicity and hepatotoxicity.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Shan S, Shen Z, Song F. Autophagy and acetaminophen-induced hepatotoxicity. Arch Toxicol. 2018; 92(7):2153–2161. doi:10.1007/s00204-018-2236-6
National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): NIDDK; 2016 [cited 2024 May 2]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548162/
Halim SZ, Abdullah NR, Afzan A, Abdul Rashid BA, Jantan I, Ismail Z. Acute toxicity study of Carica papaya leaf extract in Sprague Dawley rats. J Med Plants Res. 2011; 5(10):1867–1872. doi:10.5897/JMPR.9000271
Henneh IT, Ahlidja W, Alake J, Kwabil A, Ahmed MA, Asante BK, Adinortey MB, Ekor M, Armah FA. Ziziphus abyssinica root bark extract ameliorates paracetamol-induced liver toxicity in rats possibly via the attenuation of oxidative stress. Toxicol Rep. 2022; 9:1929–1937. doi:10.1016/j.toxrep.2022.08.017
Mohamed EA, Ahmad M, Ang LF, Asmawi MZ, Yam MF. Evaluation of the anti-inflammatory, antipyretic and analgesic activities of Orthosiphon stamineus in animal models. Mol Med Rep. 2011; 5(5):1153–1156. doi:10.3892/mmr.2011.784
Sutejo IR, Hasanah AR, Sudarko FR. Ethanolic extract of edamame (Glycine max L. Merrill) enhances second-degree burn wound healing through modulating hydroxyproline levels and increasing epithelial thickness. Acta Med Marisiensis. 2022; 68(2):55–60. doi:10.2478/amma-2021-0037
González-Montoya M, Hernández-Ledesma B, García-Nebot MJ, Herrero M, Fernández-Tomé S. Soybean Bowman-Birk inhibitor (BBI) modulates antioxidant enzyme expression via NF-κB and Nrf2 pathways in HepG2 cells. Antioxidants (Basel). 2020; 9(3):230. doi:10.3390/antiox9030230
Malhadar K, Hossain A, Islam F, Islam S, Islam MA, Shahriar M, Rahman MM. Antioxidant and hepatoprotective activity of Piper retrofractum against paracetamol-induced hepatotoxicity in Sprague-Dawley rat. Nat Prod Res. 2019; 33(11):1623–1627. doi:10.1080/14786419.2018.1428587
Pongjit K, Ninsontia C, Chaotham C, Chanvorachote P. Protective effect of Glycine max and Chrysanthemum indicum extracts against cisplatin-induced renal epithelial cell death. Hum Exp Toxicol. 2011; 30(12):1931–1944. doi:10.1177/0960327111400458
Ramasamy A, Jothivel N, Das S, Swapna A, Albert AP, Barnwal P, Babu D. Evaluation of the protective role of Glycine max seed extract (soybean oil) in drug-induced nephrotoxicity in experimental rats. J Diet Suppl. 2018; 15(5):583–595. doi:10.1080/19390211.2018.1463337
Reshi MS, Yadav D, Uthra C, Shrivastava S, Shukla S. Acetaminophen-induced renal toxicity: preventive effect of silver nanoparticles. Toxicol Res (Camb). 2020; 9(4):406–412. doi:10.1093/toxres/tfaa042
Arifin HA, Hashiguchi T, Nagahama K, Hashiguchi M, Muguerza M, Sakakibara Y, Tanaka H, Akashi R. Varietal differences in flavonoid and antioxidant activity in Japanese soybean accessions. Biosci Biotechnol Biochem. 2021; 85(4):916–922. doi:10.1093/bbb/zbaa056
Liu YT, Chen YH, Uramaru N, Lin AH, Yang HT, Lii CK, Yao HT. Soy isoflavones reduce acetaminophen-liver injury by inhibiting cytochrome P450-mediated bioactivation and glutathione depletion and increasing urinary drug excretion in rats. J Funct Foods. 2016; 26:135–143. doi:10.1016/j.jff.2016.07.007
Wisudanti DD, Normasari R, Herdiyana F. Nephroprotective effect of soy (Glycine max (L.) Merrill) flour against diazinon-induced renal toxicity in rats. Univ Med. 2020; 39(3):192–198. doi:10.18051/UnivMed.2020.v39.192-198
Zhou Y, Vaidya VS, Brown RP, Zhang J, Rosenzweig BA, Thompson KL, Miller TJ, Bonventre JV, Goering PL. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008; 101(1):159–170. doi:10.1093/toxsci/kfm260
Ozatik FY, Teksen Y, Kadioglu E, Ozatik O, Bayat Z. Effects of hydrogen sulfide on acetaminophen-induced acute renal toxicity in rats. Int Urol Nephrol. 2019; 51(4):745–754. doi:10.1007/s11255-019-02124-2
Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH. The role of tumour necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci. 2021; 22(5):2719. doi:10.3390/ijms22052719
Khodarahmi M, Foroumandi E, Jafarabadi MA. Effects of soy intake on circulating levels of TNF-α and interleukin-6: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr. 2021; 60(2):581–601. doi:10.1007/s00394-020-02305-7
Prokopidis K, Mazidi M, Sankaranarayanan R, Tajik B, McArdle A, Isanejad M. Effects of whey and soy protein supplementation on inflammatory cytokines in older adults: a systematic review and meta-analysis. Br J Nutr. 2022; 129(5):759–770. doi:10.1017/S0007114521001617
Bashandy SAE, Ebaid H, Al-Tamimi J, Hassan I, Omara EA, Elbaset MA, Alhazza IM, Siddique JA. Protective effect of daidzein against diethylnitrosamine/carbon tetrachloride-induced hepatocellular carcinoma in male rats. Biology (Basel). 2023; 12(9):1184. doi:10.3390/biology12091184
Kim IS, Yang WS, Kim CH. Beneficial Effects of Soybean-Derived Bioactive Peptides. Int J Mol Sci. 2021; 22(16):8570. https://doi.org/10.3390/ijms22168570
Lu Y, Li W, Yang X. Soluble soybean polysaccharides enhance the protective effects of genistein against hepatic injury in high l-carnitine-fed mice. Food Funct. 2017; 8(12):4364-4373. doi: 10.1039/c7fo00907k
Misiakiewicz K, Maciejewska D, Szypulska D, Kolasa A, Wiszniewska B. The Influence of Soy Isoflavones and Soy Isoflavones with Inulin on Kidney Morphology, Fatty Acids, and Associated Parameters in Rats with and without Induced Diabetes Type 2. Int J Mol Sci. 2024; 25(10):5418. doi: 10.3390/ijms25105418