Ameliorative potentials of Linum usitatissimum (Linn) (Flax seed) Crude Extract Against Lead Acetate-induced Neuronal Damage on the Hippocampal Histoarchitecture Using Wistar rat Model
Main Article Content
Abstract
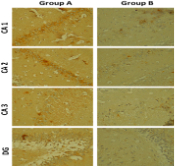
Lead-induced neurotoxicity is on the increase and the search for herbal remedy over orthodox intervention is also gaining popularity. The study investigated the ameliorative properties of flaxseed crude extract against lead acetate-induced neuronal damage in the hippocampus of Wistar rat. Thirty adult male Wistar rats (100 – 120 g) were used and assigned randomly into five groups A-E (n=6). Group A is the control, Group B was the negative control, while Groups C, D, E were test groups. Lead acetate was administered at a dose of 5 mg/kg for 14 days, Dimercaptosuccinic acid (DMSA) and flaxseed were later administered for 14 days. Neurobehavioral test (Radial arm maze) was carried out after administration. At the end of the experiment, the animals were sacrificed using cervical dislocation. Hippocampal tissues were collected after animal sacrifice and analyzed for glucose-6-phosphate dehydrogenase activity, Malondialdehyde (MDA), gamma-aminobutyric acid (GABA) levels, and the immunohistochemical expression of Tumor Necrosis Factor α (TNF-α) using enzyme tests. Data collected were analysed using one-way ANOVA, followed by Tukey post hoc for multiple comparisons. Flax seed extract reversed the neuronal damage and spatial memory deficit caused by lead acetate exposure to the hippocampus. The study concluded that 200 mg/kg of Linum usitatissimum crude extract had an ameliorative effect against memory impairment caused by lead acetate in rat model.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Ibrahim NM, Eweis EA, El-Beltagi HS, Abdel-Mobdy YE. Effect of lead acetate toxicity on experimental male albino rat. Asi Pac J of Trop Bio. 2012; 2(1), 41–46. https://doi.org/10.1016/S2221-1691(11)
Flora G, Gupta D, Tiwari A. Toxicity of lead: A review with recent updates. Interdiscip Toxicol. 2012; 5(2):47–58. 23118587.
Rezaee M, Esfahani Z, Nejadghaderi SA, Abbasi-Kangevari M, Saeedi Moghaddam S, Ghanbari A, Ghamari A, Golestani A, Foroutan Mehr E, Kazemi A, Haghshenas R. Estimating the burden of diseases attributable to lead exposure in the North Africa and Middle East region, 1990–2019: a systematic analysis for the Global Burden of Disease study 2019. Enviro Heal. 2022;21(1):105.
World Health Organization. (WHO), Fact sheets, Lead poisoning. Available on: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning- and-health. 2022.
Correia PRM, Oliveira E, Oliveira PV. Simultaneous determination of Cd and Pb in foodstuffs by electro-thermal atomic absorption spectrometry. Anal Chim Acta. 2000; 405(1–2):205–211.
Elrasoul ASA, Mousa AA, Orabi SH, Mohamed MAE, Gad-Allah SM, Almeer R, Abdel-Daim MM, Khalifa SAM, El-Seedi HR, Eldaim MAA. Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Effects of Azolla pinnata Ethanolic Extract against Lead-Induced Hepatotoxicity in Rats. Antioxidants (Basel). 2020; 9(10):1014.
Amedu ON, Omotosho GO. Lead acetate-induced neurodegenerative changes in the dorsolateral prefrontal cortex of mice: the role of Vitexin. Environ Anal Heal and Toxicol. 2020 35(1): e2020001.
Migliore L, Coppedè F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res. 2009; 674, 73–84.
Hosseinzadeh H, Sadeghnia HR, Ghaeni FA, Motamedshariaty VS, Mohajeri SA. Effects of Saffron (Crocus Sativus l.) and its Active Constituent, Crocin, on Recognition and Spatial Memory after Chronic Cerebral Hypoperfusion in Rats. Phytotherapy Res. 2012; 26, 381–386.
doi:10.1002/ptr.3566
Sidhu P, Nehru B. Lead Intoxication: Histological and Oxidative Damage in Rat Cerebrum and Cerebellum. The J T Ele. Exp. Med. 2004; 17:45-53.
Anand KS, Dhikav V. Hippocampus in health and disease: An overview. Annals of Indian Acad. Neurol. 2012; 15(4), 239.
Deveci E. Ultrastructural effects of lead acetate on brain of rats. Toxicol and Indus. Heal. 2006; 22(10), 419–422.
Abdel-Moneim AE, Dkhil MA, Al-Quraishy S. The Redox Status in Rats Treated with Flaxseed Oil and Lead-Induced Hepatotoxicity. Biol Trace Elem Res. 2011b; 143, 457–467. https://doi.org/10.1007/s12011-010-8882-z
Milder IE, Arts IC, Van de Putte B, Venema DP, Hollman PC. Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br J Nutr. 2005; 93:393–402.
Ismail AFM, Salem AAM, Eassawy MMT. Modulation of gamma-irradiation and carbon tetrachloride induced oxidative stress in the brain of female rats by flaxseed oil. J. of Photochem and Photobio. 2016; 161, 91–99. 10.1016/j.jphotobiol.2016.04.031
Touré A, Xueming X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compre. Rev. in Food Sci. and Food Saf. 2010; 9(3), 261–269.
Mazza M, Pomponi M, Janiri L, Bria P, Mazza S. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: an overview. Prog Neuropsychopharmacol Biol Psychiatry. 2007; 31:12– 26.
Uauy R, Dangour, AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev. 2006; 64:S24–S533, discussion S72-S91
Makni M, Sefi M, Fetoui H, Garoui EM, Gargouri NK, Boudawara T, Zeghal N. Flax and Pumpkin seeds mixture ameliorates diabetic nephropathy in rats. Food and Chem. Toxicol. 2010; 48(8-9), 2407-2412.
Al Za’abi M, Ali H, Ali BH. Effect of flaxseed on systemic inflammation and oxidative stress in diabetic rats with or without chronic kidney disease. Plos One. 2021; 16(10), e0258800.
Metryka E, Chibowska K, Gutowska I, Falkowska A, Kupnicka P, Barczak K, Chlubek D, Baranowska-Bosiacka I. Lead (Pb) Exposure Enhances Expression of Factors Associated with Inflammation. Int J Mol Sci. 2018; 19 (6):1813.
Miller AL. Dimercaptosuccinic acid (DMSA), a non-toxic, water-soluble treatment for heavy metal toxicity. Altern Med Rev. 1998; 3(3):199-207. PMID: 9630737.
Shaban NZ, Abd El-Kader SE, Mogahed FAK, El-Kersh MAL, Habashy NH. Synergistic protective effect of Beta vulgaris with meso 2, 3-dimercaptosuccinic acid against lead-induced neurotoxicity in male rats. Sci Rep. 2021; 11(1):252.
Flora SJ, Pachauri V. Chelation in metal intoxication. Int J Environ Res Pub. Health. 2010; 7(7):2745-88.
Ahmad N, Akhtar N, Ali S. Effects of Aqueous Methanolic Extract of Flax Seeds (Linum usitatissimum) on Serum Estradiol, Progesterone, Kidney and Liver Functions and Some Serum Biochemical Metabolites in Immature Female Rats. Pakistan Vet. J. 2012; 32(2).
Gad SC, and Pham T. Lead. Encyclo. of Toxicol. 2014; 61–65.
Friedheim E, Corvi C. Meso-dimercaptosuccinic acid, a chelating agent for the treatment of mercury poisoning. J. Pharm. Pharmac. 1975; 37, 624-626.
Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats. Journal of experimental psychology: Ani. Beh. Processes. 1976; 2(2), 97.
Levin ED. Learning about cognition risk with the radial-arm maze in the developmental neurotoxicology battery. Neurotoxicol Teratol. 2015; 52(Pt A):88-92.
Mohammad SA, Mohammad GK, Nusrat J, Ric N. P and Benedikt L (2018): Spectrophotometry assays to determine G6PD activity from Trinity Biotech and Pointe Scientifc G6PD show good correlation, BMC Res Notes, 11:855 https://doi.org/10.1186/s13104-018-3964-7
Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet (London, England). 2008; 371(9606), 64–74. https://doi.org/10.1016/S0140-6736(08)60073-2
Owolabi J, Olatunji SY, Olanrewaju J. Caffeine and Cannabis Effects on Vital Neurotransmitters and Enzymes in the Brain Tissue of Juvenile Experimental Rats. Annals of Neurosciences. 2017; 24:65-73. 10.1159/000475895.
Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014:360438.
Farombi EO, Tahnteng JG, Agboola AO, Nwankwo JO, Emerole GO. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron--a Garcinia kola seed extract. Food and chem toxico. 2000; 38(6), 535–541. https://doi.org/10.1016/s0278-6915(00)00039-9
Mei J, Kohler J, Winter Y, Spies C, Endres M, Banneke S, Emmrich JV. Automated radial 8-arm maze: A voluntary and stress-free behavior test to assess spatial learning and memory in mice. Behavioural brain research. 2020; 381, 112352. https://doi.org/10.1016/j.bbr.2019.112352
Mason LH, Harp JP, Han DY. Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int. 2014; 840547.
Yun S, Wu Y, Niu R, Feng C, Wang J. Effects of lead exposure on brain glucose metabolism and insulin signaling pathway in the hippocampus of
rats. Toxicol. letters. Aug: 310:23-30. doi: 10.1016/j.toxlet.2019.04.011.
Tiwari M. Glucose 6 phosphatase dehydrogenase (G6PD) and neurodegenerative disorders: Mapping diagnostic and therapeutic opportunities. Genes & Diseases. 2017; 4(4): 196-203.
Wirbisky SE, Weber GJ, Lee JW, Cannon JR, Freeman JL. Novel dose-dependent alterations in excitatory GABA during embryonic development associated with lead (Pb) neurotoxicity. Toxicol. letters. 2014; 229(1), 1–8. https://doi.org/10.1016/j.toxlet.2014.05.016.
Soares FA, Farina M, Santos FW et al., Interaction Between Metals and Chelating Agents Affects Glutamate Binding on Brain Synaptic Membranes. Neurochem Res. 2003; 28,1859–1865 https://doi.org/10.1023/A:1026175825871
Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006; 52, 601–623.
Lorente L, Martín MM, Abreu-González P, Ramos L, Argueso M, Cáceres JJ, Solé-Violán J, Lorenzo JM, Molina I, Jiménez A. Association between serum malondialdehyde levels and mortality in patients with severe brain trauma injury. J Neurotrauma. 2015; 32(1):1-6.
Chera EI, Pop T I, Pop RM, Pârvu M, Uifălean A, Cătoi FA, Cecan AD, Mîrza CM, Achimaș-Cadariu P, Pârvu AE. Flaxseed Ethanol Extract Effect in Acute Experimental Inflammation. Medicina (Kaunas, Lithuania). 2022; 58(5), 582. https://doi.org/10.3390/medicina58050582
Abdel-Moneim AE, Dkhil, MA, Al-Quraishy S. Effects of Flaxseed Oil on Lead Acetate-Induced Neurotoxicity in Rats. Biol. Trace Ele. Res. 2011a; 144(1-3), 904–913.
Mahmoud YI, Sayed SS. Effects of L-cysteine on lead acetate induced neurotoxicity in albino mice. Biotech & histochem. 2016; 91(5), 327–332. https://doi.org/10.3109/10520295.2016.1164897
Clark IA, Alleva LM, Vissel B. The roles of TNF in brain dysfunction and disease. Pharma & Therap. 2010; 128 (3): 519-548.
Iwata M, Ota KT, Duman RS. The inflammasome: Pathways linking psychological stress, depression, and systemic illnesses. Brain, Beh, and Immun. 2013; 31: 105-114.
Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cellular Signaling. 2010; 22(7):977-983
Takahashi S, Fukushima H, Yu Z, Tomita H, Kida S. Tumor necrosis factor α negatively regulates the retrieval and reconsolidation of hippocampus-dependent memory. Brain, Beh, and Immun. 2021; 94:79-88, https://doi.org/10.1016/j.bbi.2021.02.033.
Losif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. The J. of neurosci. 2006; 26(38), 9703–9712.