Revealing the Potential of New Immunomodulatory Agents from Katokkon Pepper as a Native Toraja Plant
Main Article Content
Abstract
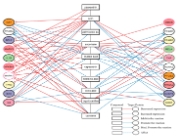
Plant bioprospecting aims to find bioactive chemicals that can be employed in various aspects of human life, including food, and medicine. The chili plant has great economic value because it is not only useful as a food ingredient but also as a medicine. Indonesia has many native chilies such as Katokkon pepper with great potential for food, and with high economic value. Katokkon pepper from Toraja have not been widely researched. This study aimed to identify the bioactive compounds in Katokkon pepper with potential immunomodulatory and anti-inflammatory activities through in-silico studies. The methods used were bioactive compounds data mining, Quantitative Structure-Activity Relationship (QSAR), drug similarity analysis, target protein prediction, gene ontology annotation, and network pharmacology. The results revealed that rutin, ascorbic acid, linoleic acid, alpha-linolenic acid, cryptoxanthin, zeaxanthin, oleic acid, palmitoleic acid, beta-carotene, and capsanthin were among the ten bioactive compounds found in Katokkon pepper that were predicted to have immunomodulatory and anti-inflammatory properties. These chemicals can modulate the expression and activities of various proteins involved in immunomodulation, inflammation, and apoptosis, including BAX, BCL2, CASP3, CAT, IKBKB, IL1B, IL6, MAPK1, MAPK3, NFE2L2, NFKBIA, PPARA, PPARB/PPARD, PPARG, PTGS2, RELA, RUNX2, SOD1, TNF, and TP53. These findings serve as critical preliminary data for further exploration of bioactive compounds from chili peppers to support human health and the food industry.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Teoh ES. Secondary Metabolites of Plants. In: Medicinal Orchids of Asia. Springer International Publishing, 2016. 59–73 doi:10.1007/978-3-319-24274-3_5.
2. Twaij BM and Hasan MN. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int J Plant Biol. 2022; 13: 4–14.
3. Saxena M, Saxena J, Nema R, Singh D, Gupta A. Phytochemistry of Medicinal Plants. J Pharmacogn Phytochem. 2013; 1(6):168-182.
4. Erb M and Kliebenstein DJ. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional
Trichotomy. Plant Physiol. 2020; 184(1):39-52. https://doi.org/10.1104/PP.20.00433.
5. Sawarkar Scholar A., Sawarkar A, Sharma R, Gautam V. Bioprospecting: Creating value for biodiversity. ~ 256 ~ Pharma Innov J. 2019; 8:256–265.
6. Beattie AJ, Hay M, Magnusson B, de Nys R, Smeathers J, Vincent JFV. Ecology and bioprospecting. Austral Ecol. 2011; 36:341–356.
7. Okoro NK, Oseni BA, Okubanjo OO, Adegun AA, Ilomuanya MO. Development of Diclofenac and Capsaicin Emulgel for the Management of Inflammation in Rheumatoid Arthritis. Trop J Nat Prod Res. 2023; 7:3246–3252.
8. Poythrees VS. Menebus Sains: Pendekatan Yang Berpusat Kepada Allah [Redeeming Science: A God-Centered Approach]. Momentum, Surabaya, 2013.
9. Djarwaningsih T. Capsicum spp. (Chili): origin, distribution, and its economical value. Biodiversitas: J Biol Divers. 2005; 6:292–296.
10. Tammu R, Daryono B, Nuringtyas T. Phenotypic and genotypic characters of Katokkon chili (Capsicum annuum L.) polyploidized with colchicine. (Universitas Gadjah Mada, 2016).
11. Center for Plant Variety Protection and Agricultural Licensing. Official News of Local Variety Registration. http://pvtpp.setjen.pertanian.go.id/berita-resmi/pendaftaran-varietas-lokal/padi-nama-varietas-katokkon/ (2014). 12. Center for Plant Variety Protection and Agricultural Licensing. Official News Local Variety Registration Publication Number 96/BR/PVL/08/2017. at http://pvtpp.setjen.pertanian.go.id/cms/wp-content/uploads/2017/12/96.-Cabai-Katokkon-Sayang.pdf (2017).
13. Amaliah N. Determination of Capsaicin Levels Using the Thin Layer Chromatography (Klt) Method in Katokkon Chili. J. Sains Terap. 2018; 4:49–56.
14. Anaya-Esparza LM, Mora ZV, Vázquez-Paulino O, Ascencio F, Villarruel-López A. Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules 2021; 26(17):5341.
15. Anjani G, Ayustaningwarno F, Eviana R. Critical review on the immunomodulatory activities of carrot’s β-carotene and other bioactive compounds. J Funct Foods 2022; 99:105303.
16. Zebeaman M, Tadesse MG, Bachheti RK, Bachheti A, Gebeyhu R, Chaubey KK. Plants and Plant-Derived Molecules as Natural Immunomodulators. BioMed Res Int. 2023; 2023:7711297.
17. Kaur M, Mahanty J, Kumar S, Sharma, A. Exploring phytochemicals as novel immunomodulators. Pharmaspire 2020; 12:44–49.
18. Mesaik MA, Jabeen A, Halim SA, Begum A, Khalid AS, Asif M, Fatima B, Ul-Haq Z and Choudhary MI. In Silico and In Vitro Immunomodulatory Studies on Compounds of Lindelofia stylosa. Chem Biol Drug Des. 2012; 79:290–299.
19. Chandran U and Patwardhan B. Network ethnopharmacological evaluation of the immunomodulatory activity of Withania somnifera. J Ethnopharmacol. 2017; 197:250–256.
20. Indradi RB, Pitaloka DAE, Suryani S. Network pharmacology to uncover potential anti-inflammatory and immunomodulatory constituents in Curcuma longa rhizome as complementary treatment in COVID-19. Pharmacia 2022; 69:995–1003.
21. Krisnamurti GC, Sari DRT, Bare Y. Capsaicinoids from Capsicum annuum as an Alternative FabH Inhibitor of Mycobacterium Tuberculosis: In Silico Study. Makara J Sci. 2021; 25:195–202.
22. Jin Z, Du X, Xu Y, Al, E. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020; 582:289–293.
23. Lagunin A, Filimonov D, Poroikov V. Multi-Targeted Natural Products Evaluation Based on Biological Activity Prediction with PASS. Curr Pharm Des. 2010; 16:1703–1717.
24. Drwal MN, Banerjee P, Dunkel M, Wettig MR, Preissner R. ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucl Acids Res. 2014; 42:W53–W58.
25. Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucl Acids Res. 2018; 46:W257–W263.
26. Dong J, Wang N-N, Yao Z-J, Zhang L, Cheng Y, Ouyang D, Lu A-P, Cao D-S. ADMETlab: a platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J Cheminform. 2018; 10:29.
27. Xiong G, Wu Z, Yi J, Fu L, Yang Z, Hsieh C, Yin M, Zeng X, Wu C, Lu A, Chen X, Hou T, Cao D. ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucl Acids Res. 2021; 49:W5–W14.
29. Sherman BT, Hao M, Qiu J, Jiao X, Baseler MW, Lane HC, Imamichi T, Chang W. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucl Acids Res. 2022; 50:W216–W221.
30. Kuhn M, Szklarczyk D, Pletscher-Frankild S, Blicher TH, von Mering C, Jensen LJ, Bork P. STITCH 4: integration of protein–chemical interactions with user data. Nucl Acids Res. 2014; 42:D401–D407.
31. Pathi SS, Lei P, Sreevalsan S, Chadalapaka G, Jutooru I, Safe S. Pharmacologic Doses of Ascorbic Acid Repress Specificity Protein (Sp) Transcription Factors and Sp-Regulated Genes in Colon Cancer Cells. Nutr Cancer. 2011; 63:1133–1142.
32. Salerno C, Capuozzo E, Crifò C, Siems W. α-Tocopherol increases caspase-3 up-regulation and apoptosis by β-carotene cleavage products in human neutrophils. Biochim Biophys Acta - Mol Basis Dis. 2007; 1772:1052–1056.
33. Abhilash PA, Harikrishnan R, Indira M. Ascorbic acid suppresses endotoxemia and NF-κB signaling cascade in alcoholic liver fibrosis in guinea pigs: A mechanistic approach. Toxicol Appl Pharmacol. 2014; 274:215–224.
34. Feng N-H, Chu S-J, Wang D, Hsu K, Lin C-H, Lin H-I. Effects of various antioxidants on endotoxin-induced lung injury and gene expression: mRNA expressions of MnSOD, interleukin-1beta and iNOS. Chin J Physiol. 2004; 47:111–120.
35. Yfanti C, Fischer CP, Nielsen S, Åkerström T, Nielsen AR, Veskoukis AS, Kouretas D, Lykkesfeldt J, Pilegaard H, Pedersen BK. Role of vitamin C and E supplementation on IL-6 in response to training. J Appl Physiol. 2012; 112:990–1000.
36. Temu TM, Wu K-Y, Gruppuso PA, Phornphutkul C. The mechanism of ascorbic acid-induced differentiation of ATDC5 chondrogenic cells. Am J Physiol Metab. 2010; 299:E325–E334.
37. Liu CM, Sun YZ, Sun JM, Ma J-Q, Cheng C. Protective role of quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-κB pathway. Biochim Biophys Acta - Gen Subj. 2012; 1820: 1693–1703.
38. Campo GM, Avenoso A, Campo S, D’Ascola A, Traina P, Samà D, Calatroni A. NF‐kB and caspases are involved in the hyaluronan and chondroitin‐4‐sulphate‐exerted antioxidant effect in fibroblast cultures exposed to oxidative stress. J Appl Toxicol. 2008; 28:509–517.
39. Ramos G, Limon-Flores AY, Ullrich SE. JP-8 Induces Immune Suppression via a Reactive Oxygen Species NF-κβ–Dependent Mechanism. Toxicol Sci. 2009; 108:100–109.
40. Yang B, Ye Z, Wang Y, Guo H, Lehmler H-J, Huang R, Song E, Song Y. Evaluation of Early Biomarkers of Atherosclerosis Associated with Polychlorinated Biphenyl Exposure: An in Vitro and in Vivo Study. Environ Health Perspect. 2022; 130(3):037011
41. Mo S-J, Son E-W, Rhee D-K, Pyo S. Modulation of tnf-α-induced icam-1 expression, no and h202 production by alginate, allicin and ascorbic acid in human endothelial cells. Arch Pharm Res. 2003; 26:244–251.
42. Ahmad J, Ahamed M, Akhtar MJ, Alrokayan SA, Siddiqui MA, Musarrat J, Al-Khedhairy AA. Apoptosis induction by silica nanoparticles mediated through reactive oxygen species in human liver cell line HepG2. Toxicol Appl Pharmacol. 2012; 259:160–168.
43. Marcil V, Delvin E, Sane A, Tremblay A, Levy E. Oxidative stress influences cholesterol efflux in THP-1 macrophages: Role of ATP-binding cassette A1 and nuclear factors☆. Cardiovasc Res. 2006; 72:473–482.
44. Gaikwad S, Chakraborty A, Salwe S, Patel V, Kulkarni S, Banerjee S. Juglone–ascorbic acid synergy inhibits metastasis and induces apoptotic cell death in poorly differentiated thyroid carcinoma by perturbing SOD and catalase activities. J Biochem Mol Toxicol. 2018; 32(9):e22176..
45. Li Y, Darwish WS, Chen Z, Hui T, Wu Y, Hirotaka S, Chiba H, Hui S-P. Identification of lead-produced lipid hydroperoxides in human HepG2 cells and protection using rosmarinic and ascorbic acids with a reference to their regulatory roles on Nrf2-Keap1 antioxidant pathway. Chem Biol Interact. 2019; 314:108847.
46. Olalekan Lawal A, Folusho Lawal A, Ologundudu A, Yakubu Adeniran O, Omonkhua A, Obi F. Antioxidant effects of heated garlic juice on cadmium-induced liver damage in rats as compared to ascorbic acid. J Toxicol Sci. 2011; 36:549–557.
47. Zhang X, Chen K, Wei B, Liu X, Lei Z, Bai X. Ginsenosides Rg3 attenuates glucocorticoid-induced osteoporosis through regulating BMP-2/BMPR1A/Runx2 signaling pathway. Chem Biol Interact. 2016; 256:188–197.
48. Wang D, Yang X, Chen Y, Gong K, Yu M, Gao Y, Wu X, Hu H, Liao C, Han J, Duan Y. Ascorbic acid enhances low-density lipoprotein receptor expression by suppressing proprotein convertase subtilisin/kexin 9 expression. J Biol Chem. 2020; 295:15870–15882.
49. Ju Q, Li X, Zhang H, Yan S, Li Y, Zhao Y. NFE2L2 Is a Potential Prognostic Biomarker and Is Correlated with Immune Infiltration in Brain Lower Grade Glioma: A Pan-Cancer Analysis. Oxid Med Cell Longev. 2020; 2020:1–26.
50. Kim WJ, Shin HL, Kim BS, Kim HJ, Ryoo HM. RUNX2-modifying enzymes: therapeutic targets for bone diseases. Exp Mol Med. 2020; 52:1178–1184.
51. Bunton-Stasyshyn RKA, Saccon RA, Fratta P, Fisher EMC. SOD1 Function and Its Implications for Amyotrophic Lateral Sclerosis Pathology. Neurosci. 2015; 21:519–529.
52. Sorice A, Guerriero E, Capone F, Colonna G, Castello G, Costantini S. Ascorbic Acid: Its Role in Immune System and Chronic Inflammation Diseases. Mini-Rev Med Chem. 2014; 14: 44–452.
53. Kim JS, Lee WM, Rhee HC, Kim S. Red paprika (Capsicum annuum L.) and its main carotenoids, capsanthin and β-carotene, prevent hydrogen peroxide-induced inhibition of gap-junction intercellular communication. Chem Biol Interact. 2016; 254:146–155.
54. Shanmugham V and Subban, R. Capsanthin fromCapsicum annum fruits exerts anti‐glaucoma, antioxidant, anti‐inflammatory activity, and corneal pro‐inflammatory cytokine gene expression in a benzalkonium chloride‐induced rat dry eye model. J Food Biochem. 2022; 46(10):e14352.
55. Al-Wadei HAN, Takahashi T, Schuller HM. Growth stimulation of human pulmonary adenocarcinoma cells and small airway epithelial cells by β-carotene via activation of cAMP, PKA, CREB and ERK1/2. Int J Cancer 2006; 118:1370–1380.
56. Zhu,X, Zhang Y, Li Q, Yang L, Zhang N, Ma S, Zhang K, Song J, Guan F. β‐Carotene Induces Apoptosis in Human Esophageal Squamous Cell Carcinoma Cell Lines via the Cav‐1/AKT/NF‐κB Signaling Pathway. J Biochem Mol Toxicol. 2016; 30:148–157.
57. Das R, Das A, Roy A, Kumari U, Bhattacharya S, Haldar PK. β-Carotene Ameliorates Arsenic-Induced Toxicity in Albino Mice. Biol Trace Elem Res. 2015; 164:226–233.
58. Chan S-T, Chuang C-H, Yeh C-L, Liao J-W, Liu K-L, Tseng M-J, Yeh S-L. Quercetin supplementation suppresses the secretion of pro-inflammatory cytokines in the lungs of Mongolian gerbils and in A549 cells exposed to benzo[a]pyrene alone or in combination with β-carotene: in vivo and ex vivo studies. J Nutr Biochem. 2012; 23: 179–185.
59. Chew BP. Role of Carotenoids in the Immune Response. J Dairy Sci. 1993; 76:2804–2811.
60. Liu XR, Wang Y-Y, Dan X-G, Kumar A, Ye T-Z, Yu Y-Y, Yang L-G. Anti-inflammatory potential of β-cryptoxanthin against LPS-induced inflammation in mouse Sertoli cells. Reprod Toxicol. 2016; 60:148–155.
61. Nishi K, Muranaka A, Nishimoto S, Kadota A, Sugahara T. Immunostimulatory effect of β-cryptoxanthin in vitro and in vivo. J Funct Foods 2012; 4: 618–625.
62. Uchiyama S and Yamaguchi M. β‐cryptoxanthin stimulates cell differentiation and mineralization in osteoblastic MC3T3‐E1 cells. J Cell Biochem. 2005; 95: 1224–1234.
63. Meerarani P, Ramadass P, Toborek M, Bauer H.-C, Bauer H, Hennig B. Zinc protects against apoptosis of endothelial cells induced by linoleic acid and tumor necrosis factor α. Am J Clin Nutr. 2000; 71:81–87.
64. Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential Activation of Nuclear Receptors by Perfluorinated Fatty Acid Analogs and Natural Fatty Acids: A Comparison of Human, Mouse, and Rat Peroxisome Proliferator-Activated Receptor-α, -β, and -γ, Liver X Receptor-β, and Retinoid X Receptor-α. Toxicol Sci. 2006; 92:476–489.
65. Meilhac O, Zhou M, Santanam N, Parthasarathy S. Lipid peroxides induce expression of catalase in cultured vascular cells. J Lipid Res. 2000; 41:1205–1213.
66. Zouboulis CC, Angres S, Seltmann H. Regulation of stearoyl-coenzyme A desaturase and fatty acid delta-6 desaturase-2 expression by linoleic acid and arachidonic acid in human sebocytes leads to enhancement of proinflammatory activity but does not affect lipogenesis. Br J Dermatol. 2011; 165:269–276.
67. Vecchini A, Ceccarelli V, Susta F, Caligiana P, Orvietani P, Binaglia L, Nocentini G, Riccardi C, Calviello G, Palozza P, Maggiano N, Di Nardo P. Dietary α-linolenic acid reduces COX-2 expression and induces apoptosis of hepatoma cells. J Lipid Res. 2004; 45:308–316.
68. Bourebaba L, Łyczko J, Alicka M, Bourebaba N, Szumny A, Fal A, Marycz K. Inhibition of Protein-Tyrosine Phosphatase PTP1B and LMPTP Promotes Palmitate/Oleate-Challenged HepG2 Cell Survival by Reducing Lipoapoptosis, Improving Mitochondrial Dynamics and Mitigating Oxidative and Endoplasmic Reticulum Stress. J Clin Med. 2020; 9:1294.
69. Vidyashankar S, Sandeep Varma R, Patki PS. Quercetin ameliorate insulin resistance and up-regulates cellular antioxidants during oleic acid induced hepatic steatosis in HepG2 cells. Toxicol in Vitro. 2013; 27:945–953.
70. Qu L, Yu B, Li Z, Jiang W, Jiang J, Kong W. Gastrodin Ameliorates Oxidative Stress and Proinflammatory Response in Nonalcoholic Fatty Liver Disease through the AMPK/Nrf2 Pathway. Phyther Res. 2016; 30:402–411.
71. Romualdo GR, Da Silva TC, de Albuquerque Landi MF, Morais JÁ, Barbisan LF, Vinken M, Oliveira CP, Cogliati B. Sorafenib reduces steatosis‐induced fibrogenesis in a human 3D co‐culture model of non‐alcoholic fatty liver disease. Environ Toxicol. 2021; 36:168–176.
72. McIlwraith EK, Loganathan N, Belsham DD. Phoenixin Expression Is Regulated by the Fatty Acids Palmitate, Docosahexaenoic Acid and Oleate, and the Endocrine Disrupting Chemical Bisphenol A in Immortalized Hypothalamic Neurons. Front Neurosci. 2018; 12:838.
73. de Souza CO, Valenzuela CA, Baker EJ, Miles EA, Rosa Neto JC, Calder PC. Palmitoleic Acid has Stronger Anti‐Inflammatory Potential in Human Endothelial Cells Compared to Oleic and Palmitic Acids. Mol Nutr Food Res. 2018; 62(10):e1800322.
74. Bermúdez MA, Pereira L, Fraile C, Valerio L, Balboa MA, Balsinde J. Roles of Palmitoleic Acid and Its Positional Isomers, Hypogeic and Sapienic Acids, in Inflammation, Metabolic Diseases and Cancer. Cells. 2022; 11:2146.
75. Ding Y, Ren K, Dong H, Song F, Chen J, Guo Y, Liu Y, Tao W, Zhang Y. Flavonoids from persimmon (Diospyros kaki L.) leaves inhibit proliferation and induce apoptosis in PC-3 cells by activation of oxidative stress and mitochondrial apoptosis. Chem Biol Interact. 2017; 275:210–217.
76. Sadowska-Woda I, Popowicz D, Karowicz-Bilińska A. Bifenthrin-induced oxidative stress in human erythrocytes in vitro and protective effect of selected flavonols. Toxicol in Vitro. 2010; 24:460–464.
77. Bispo da Silva A, Cerqueira Coelho PL, Alves Oliveira Amparo J, Alves de Almeida Carneiro MM, Pereira Borges JM, dos Santos Souza C, Dias Costa MF, Mecha M, Guaza Rodriguez C, Amaral da Silva VD, Lima Costa S. The flavonoid rutin modulates microglial/macrophage activation to a CD150/CD206 M2 phenotype. Chem Biol Interact. 2017; 274:89–99.
78. Potapovich AI, Lulli D, Fidanza P, Kostyuk VA, De Luca C, Pastore S, Korkina LG. Plant polyphenols differentially modulate inflammatory responses of human keratinocytes by interfering with activation of transcription factors NFκB and AhR and EGFR–ERK pathway. Toxicol Appl Pharmacol. 2011; 255: 138–149.
79. Wu C, Wu C, Huang H, Jao Y, Yen G. Naturally occurring flavonoids attenuate high glucose‐induced expression of proinflammatory cytokines in human monocytic THP‐1 cells. Mol Nutr Food Res. 2009; 53:984–995.
80. Ferruelo A, Romero I, Cabrera PM, Arance I, Andrés G, Angulo JC. Effects of resveratrol and other wine polyphenols on the proliferation, apoptosis and androgen receptor expression in LNCaP cells. Actas Urológicas Españolas 2014; 38:397–404.
81. Domitrović R, Jakovac H, Vasiljev Marchesi V, Vladimir-Knežević S, Cvijanović O. Tadić Ž, Romić Ž, Rahelić D.
Differential hepatoprotective mechanisms of rutin and quercetin in CCl4-intoxicated BALB/cN mice. Acta Pharmacol Sin. 2012; 33:1260–1270.
82. Ganeshpurkar A and Saluja AK. Protective effect of rutin on humoral and cell mediated immunity in rat model. Chem Biol Interact. 2017; 273:154–159.
83. Yoo H, Ku S-K, Baek Y-D, Bae J-S. Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflamm Res. 2014; 63:197–206.
84. Amengual J, Lobo GP, Golczak M, Li HNM, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011; 25:948–959.
85. Xiao J, Wang J, Xing F, Han T, Jiao R, Liong EC, Fung M-L, So K-F, Tipoe GL. Zeaxanthin Dipalmitate Therapeutically Improves Hepatic Functions in an Alcoholic Fatty Liver Disease Model through Modulating MAPK Pathway. PLoS One 2014; 9:e95214.
86. Jin X, Jin W, Li G, Zheng J. Zeaxanthin attenuates OVA-induced allergic asthma in mice by regulating the p38 MAPK/β-catenin signaling pathway. Allergol Immunopathol. 2022; 50:75–83.
87. Yamaguchi M and Uchiyama S. Combination of beta-cryptoxanthin and zinc has potent effects on apoptotic cell death and suppression of bone resorption-related gene expression in osteoclastic cells. Int J Mol Med. 2008; 22:221–228.