Optimization of Solvent Mixtures and Maceration Method Using Simplex Centroid Design for Phenolic Extraction and Radical Scavenging Activity in Amomum compactum Fruit
Main Article Content
Abstract
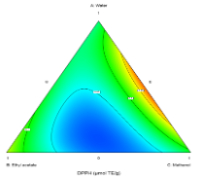
Phenolic compounds are valued for their antioxidant, anti-inflammatory, and antimicrobial properties, making them crucial in food, pharmaceutical, and nutraceutical industries. This study aimed to optimize phenolic antioxidant extraction from Amomum compactum fruit using a simplex-centroid design with water, methanol, ethanol, and ethyl acetate as solvents. The maceration process, applied to 15 solvent combinations, yielded total phenolic content (TPC) values ranging from 0.045 to 0.346 mg GAE/g DW and DPPH radical scavenging activities from 0.001 to 0.197 μmol TE/g DW. The optimal solvent mixture, achieving a desirability score of 0.933, was a 50:50 mixture of water and methanol, resulting in a TPC of 0.333 mg GAE/g DW and DPPH antioxidant activity of 0.179 μmol TE/g DW. The high polarity of methanol, particularly in combination with water, enhances the extraction of phenolic and antioxidant compounds. In contrast, ethyl acetate demonstrated a lower efficiency, with the lowest TPC (0.045 mg GAE/g DW) and DPPH activity (0.001 μmol TE/g DW) observed in combinations containing this solvent. These findings underscore the critical role of solvent polarity and interactions in optimizing the extraction of bioactive compounds, providing valuable insights for applications in food and pharmaceutical industries.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Sun W, Shahrajabian MH. Therapeutic potential of phenolic compounds in medicinal plants—natural health products for human health. Molecules. 2023;28:1845. doi:10.3390/molecules28041845.
Seno DSH, Sembiring DPDB, Kurniatin PA, Nurcholis W. Total phenolic, total flavonoid contents, and radical scavenging activity of different concentrations of ethanol extract of Pyrrosia piloselloides (L.) M. G. Price. Trop J Nat Prod Res. 2022;6:484–487. doi:10.26538/tjnpr/v6i4.5.
Ganga Rao B, Umamaheswara Rao P, Sambasiva Rao E, Mallikarjuna Rao T, Praneeth DVS. Studies on phytochemical constituents, quantification of total phenolic, alkaloid content, and in-vitro antioxidant activity of Thespesia populnea seeds. Free Radic Antioxid. 2011;1:56–61. doi:10.5530/ax.2011.4.9.
Belwal T, Dhyani P, Bhatt ID, Rawal RS, Pande V. Optimization of extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016;207:115–124. doi:10.1016/j.foodchem.2016.03.081.
Upadhya V, Pai SR, Hegde HV. Effect of method and time of extraction on total phenolic content in comparison with antioxidant activities in different parts of Achyranthes aspera. J King Saud Univ Sci. 2015;27:204–208. doi:10.1016/j.jksus.2015.04.004.
Arroy JDV, Ruiz-Espinosa H, Luna-Guevara JJ, Luna-Guevara ML, Hernández-Carranza P, Ávila-Sosa R, et al. Effect of solvents and extraction methods on total anthocyanins, phenolic compounds, and antioxidant capacity of Renealmia alpinia (Rottb.) Maas peel. Czech J Food Sci. 2017;35:456–465. doi:10.17221/316/2016-CJFS.
Ćujić N, Šavikin K, Janković T, Pljevljakušić D, Zdunić G, Ibrić S. Optimization of polyphenols extraction from dried chokeberry using maceration as a traditional technique. Food Chem. 2016;194:135–142. doi:10.1016/j.foodchem.2015.08.008.
Ashokkumar K, Murugan M, Dhanya MK, Warkentin TD. Botany, traditional uses, phytochemistry, and biological activities of cardamom [Elettaria cardamomum (L.) Maton]—a critical review. J Ethnopharmacol. 2020;246:112244. doi:10.1016/j.jep.2019.112244.
Mahfur M, Khasanah K, Anindhita MA, Chandra SG, Hidayah AN. Comparison of the total phenolic and flavonoid contents of Amomum compactum Sol. ex Maton from districts Linggo Asri and Paninggaran, Pekalongan Regency. Sasambo J Pharm. 2023;4:8–13. doi:10.29303/sjp.v4i1.212.
Pérez-Jiménez J, Díaz-Rubio ME, Saura-Calixto F. Non-extractable polyphenols, a major dietary antioxidant: occurrence, metabolic fate, and health effects. Nutr Res Rev. 2013;26:118–129. doi:10.1017/S0954422413000097.
Bano S, Ahmad N, Sharma AK. Phytochemical screening and evaluation of anti-microbial and antioxidant activity of Elettaria cardamomum (cardamom). J Appl Nat Sci. 2016;8:1966–1970. doi:10.31018/jans.v8i4.1071.
Mussatto SI, Ballesteros LF, Martins S, Teixeira JA. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep Purif Technol. 2011;83:173–179. doi:10.1016/j.seppur.2011.09.036.
Oussaid S, Chibane M, Madani K, Amrouche T, Achat S, Dahmoune F, et al. Optimization of the extraction of phenolic compounds from Scirpus holoschoenus using a simplex centroid design for antioxidant and antibacterial applications. LWT- Food Sci Technol. 2017;86:635–642. doi:10.1016/j.lwt.2017.08.064.
Aazza S. Application of multivariate optimization for phenolic compounds and antioxidants extraction from Moroccan Cannabis sativa waste. J Chem. 2021;2021:1–11. doi:10.1155/2021/9738656.
Nurcholis W, Marliani N, Asyhar R, Minarni M. Optimized solvents for the maceration of phenolic antioxidants from Curcuma xanthorrhiza rhizome using a simplex centroid design. J Pharm Bioallied Sci. 2023;15:35–41. doi:10.4103/jpbs.jpbs_185_23.
Fadil M, Lebrazi S, Aboulghazi A, Guaouguaou FE, Rais C, Slimani C, et al. Multi-response optimization of extraction yield, total phenols-flavonoids contents, and antioxidant activity of extracts from Moroccan Lavandula stoechas leaves: predictive modeling using simplex-centroid design. Biocatal Agric Biotechnol. 2022;43:102430. doi:10.1016/j.bcab.2022.102430.
Marliani N, Artika IM, Nurcholis W. Optimization extraction for total phenolic, flavonoid contents, and antioxidant activity with different solvents and UPLC-MS/MS metabolite profiling of Justicia gendarussa Burm.f. Chiang Mai Univ J Nat Sci. 2022;21:e2022046. doi:10.12982/CMUJNS.2022.046.
de Morais Sousa M, de Lima A, Araujo BQ, dos Santos Rocha M, dos Santos Monção Filho E, de Sousa RP, et al. Multi-response optimization of a solvent system for the extraction of antioxidant polyphenols from Syzygium cumini (L.) Skeels. Food Anal Methods. 2022;15:34–45. doi:10.1007/s12161-021-02087-0.
Wan-Ibrahim WI, Sidik K, Kuppusamy UR. A high antioxidant level in edible plants is associated with genotoxic properties. Food Chem. 2010;122:1139–1144. doi:10.1016/j.foodchem.2010.03.101.
Meneses NGT, Martins S, Teixeira JA, Mussatto SI. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep Purif Technol. 2013;108:152–158. doi:10.1016/j.seppur.2013.02.015.
Ozbek Yazici S, Ozmen İ. Ultrasound-assisted extraction of phenolic compounds from Capparis ovata var. canescens fruit using deep eutectic solvents. J Food Process Preserv. 2022;46:e16286. doi:10.1111/jfpp.16286.
Galanakis CM, Goulas V, Tsakona S, Manganaris GA, Gekas V. A knowledge base for the recovery of natural phenols with different solvents. Int J Food Prop. 2013;16:382–396. doi:10.1080/10942912.2010.522750.
Alara OR, Abdurahman NH, Ukaegbu CI. Extraction of phenolic compounds: a review. Curr Res Food Sci. 2021;4:200–214. doi:10.1016/j.crfs.2021.03.011.
Rajauria G, Jaiswal AK, Abu-Gannam N, Gupta S. Antimicrobial, antioxidant, and free radical-scavenging capacity of brown seaweed Himanthalia elongata from the western coast of Ireland. J Food Biochem. 2013;37:322–335. doi:10.1111/j.1745-4514.2012.00663.x.
Moayyed M, Eftekhharian AR, Fereiduni MS, Akbary P. Effect of solvent type on phytochemical properties of burdock (Arctium lappa) extract and their effect on some pathogenic bacteria strains in rainbow trout, Oncorhynchus mykiss. Iran J Fish Sci. 2023;22:493–510. doi:10.22092/ijfs.2023.129171.
Sarikurkcu C, Ozer MS, Tepe B, Dilek E, Ceylan O. Phenolic composition, antioxidant, and enzyme inhibitory activities of acetone, methanol, and water extracts of Clinopodium vulgare L. subsp. vulgare L. Ind Crops Prod. 2015;76:961–966. doi:10.1016/j.indcrop.2015.08.011.
Namvar K, Salehi EA, Mokhtarian N. Total phenolic compounds and antioxidant activity of Stachys turcomanica. Biosci J. 2018:1349–1356. doi:10.14393/BJ-v34n5a2018-41517.
Chua LS, Hidayathulla S, Chandrashekar KR. Phytochemical evaluation, antioxidant, and antibacterial activity of Hopea ponga (Dennst.) Mabberly extracts. Int J Pharm Pharm Sci. 2014;6:326–330.
Li H, Hao Z, Wang X, Huang L, Li J. Antioxidant activities of extracts and fractions from Lysimachia foenum-graecum Hance. Bioresour Technol. 2009;100:970–974. doi:10.1016/j.biortech.2008.07.021.