Genotyping and Phytochemical Analysis of Three Species of Spider Lily (Hymenocallis spp.)
Main Article Content
Abstract
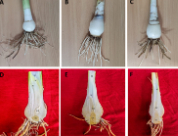
Spider lily is a plant that contains phytochemicals for various pharmacological activities. Morphological similarities in several spider lily species make it difficult to identify morphologically. Molecular approaches are recommended for identifying plant species that are difficult to identify morphologically. This study aimed to identify three species of spider lily (Hymenocallis spp.) in Denpasar, Bali (Flat Lily, Bangkok Lily, and Small Leaf Lily) using DNA barcoding. The phytochemical of the extract was identified using Gas Chromatography-Mass Spectrometry (GC-MS). The molecular data showed that the three species were genetically close to Hymenocallis littoralis, although significant genetic distance was still found. Based on phylogenetic tree construction using the Maximum Likelihood method, the three species in Denpasar are in the same clade as Hymenocallis littoralis, Hymenocallis maximiliani, and Hymenocallis caribaea but still show some relevant genetic variation. Various compounds were identified in the leaf extracts. 2,4,6-Cycloheptatrien, Trehalose and Hydroxymethylfurfural were bioactive compounds with high AUC known with known antioxidant, anti-inflammatory, and neuroprotective potential.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Huda-Shakirah AR, Kee YJ, Hafifi AB, Mohamad Azni NN, Zakaria L, Mohd MH. Identification and characterization of Macrophomina phaseolina causing leaf blight on white spider Lilies (Crinum asiaticum and Hymenocallis littoralis) in Malaysia. Mycobiol. 2019;47(4):408-414.
2. Idso SB, Kimball BA, Pettit GR, Garner LC, Backhaus RA. Effects of Atmospheric CO2 Enrichment on the Growth and Development of Hymenocallis littoralis (Amaryllidaceae) and the Concentrations of Several Antineoplastic and Antiviral Constituents of Its Bulbs. Am J Bot. 2000;87(6):769-773. doi:10.2307/2656884
3. Okazaki Y. Edible Lily Bulb Modulates Colonic Barrier Functions, Microflora and Fermentation in Rats Fed a High-Fat Diet. J Nutr Heal Food Sci. 2014;2(1):1-7. doi:10.15226/jnhfs.2014.00112
4. Nadiah MAN, Nor NMIM, Zakaria L, Hawa MM. First Report of Leaf Blight on White Spider Lily Caused by Neoscytalidium dimidiatum in Malaysia. New Dis Reports. 2017;35(1):16. doi:10.5197/j.2044-0588.2017.035.016
5. Tyagi K, Kumar V, Kundu S, Pakrashi A, Prasad P, Caleb JT, Chandra K. Identification of Indian Spiders Through DNA Barcoding: Cryptic Species and Species Complex. Sci Rep. 2019;9(1):1-13. doi:10.1038/s41598-019-50510-8
6. Wirawan IGP, Vernandes Sasadara MM, Wijaya IN, Krinandika AAK. DNA barcoding in molecular identification and phylogenetic relationship of beneficial wild Balinese red algae, Bulung sangu (Gracilaria sp.). Bali Med J. 2020;10(1):82-88. doi:10.15562/bmj.v10i1.2093
7. Pv C, B HN, Sy C, Ganapathi M, Kantharaj Y. Standardization of Plant Growth Regulators on Growth and Flowering of Spider Lily (Hymenocallis speciosa L.). Int J Chem Stud. 2020;8(5):1748-1751. doi:10.22271/chemi.2020.v8.i5x.10550
8. Cui Z. Integrated Transcriptome and Metabolome Revealed the Drought Responsive Metabolic Pathways in Oriental Lily (Lilium L.). Peer J. 2023;11:e16658,1-18. doi:10.7717/peerj.16658
9. Wei S, Luo Z, Cui S, Qiao J, Zhang Z, Zhang L, Fu J, Ma X. Molecular Identification and Targeted Quantitative Analysis of Medicinal Materials From Uncaria Species by DNA Barcoding and LC-MS/MS. Molecules. 2019;24(1):175,1-14. doi:10.3390/molecules24010175
10. Kúdelová T. DNA Barcoding of Black Flies (Diptera: Simuliidae) in Slovakia and Its Utility for Species Identification. Diversity. 2023;15(5):661,1-17. doi:10.3390/d15050661
11. Lin M, Wang H, Yu Q, Wang D. A Sensitive and Robust DNA Method for Authenticity Determination of Glehnia littoralis and Its Food Products. Res Sq. 2022; Jan:1-16. doi:10.21203/rs.3.rs-2361738/v1
12. Xin T, Li R, Lou Q, Lin Y, Liao H, Sun W, Guan M, Zhou J, Song J. Phytomedicine Application of DNA barcoding to the entire traditional Chinese medicine industrial chain : A case study of Rhei Radix et Rhizoma. Phytomedicine. 2022;105:154375. doi:10.1016/j.phymed.2022.154375
13. Braukmann T, Kuzmina ML, Sills J, Zakharov E, Hebert PDN. Testing the Efficacy of DNA Barcodes for Identifying the Vascular Plants of Canada. PLoS One. 2017;12(1):e0169515,1-19. doi:10.1371/journal.pone.0169515
14. Li Y, Gao L, Poudel RC, D L. High universality of matK primers for barcoding gymnosperms. J Syst Evol. 2011;49(3):169-175. doi:10.1111/j.1759-6831.2011.00128.x
15. Girma G, Spillane C, Gedil M. DNA Barcoding of the Main Cultivated Yams and Selected Wild Species in the Genus Dioscorea. J Syst Evol. 2015;54(3):228-237. doi:10.1111/jse.12183
16. Alves TLS, Chauveau O, Eggers L, Souza-Chies TT. Species discrimination in Sisyrinchium (Iridaceae): assessment of DNA barcodes in a taxonomically challenging genus. Mol Ecol Resour. 2013;14(2):324-335. doi:10.1111/1755-0998.12182
17. Sundari S, Mas’ud A, Arumingtyas EL, Wahyudi D. Using Short Sequence Matk Gene As Barcode DNA For Identification of Durio sp In Ternate Island. J Biosilampari J Biol. 2022;5(1):50-56. doi:10.31540/biosilampari.v5i1.1528
18. Cahyaningsih R, Compton L, Rahayu R, Brehm JM, Maxted N. DNA Barcoding Medicinal Plant Species From Indonesia. Plants. 2022;11(10):1375,1-22. doi:10.3390/plants11101375
19. Boro H, Usha T, Babu D, Chandana P, Goyal AK, Ekambaram H, Yusufoğlu HS, Das S, Middha SK. Hepatoprotective Activity of the Ethanolic Extract of Morus indica Roots From Indian Bodo Tribes. Sn Appl Sci. 2022;4(2):1-14. doi:10.1007/s42452-021-04859-z
20. Shad N, Javaid A, Kanwal Q. Antifungal And Other Bioactive Constituents In Roots Of A Halophytic Weed Suaeda fruticosa. J Weed Sci Res. 2022;28(3):311-318. doi:10.28941/pjwsr.v28i3.1072
21. Cao F, Orth C, Donlin MJ, Adegboyega PA, Meyers MJ, Murelli RP, Elagawany M, Elgendy B, Tavis JE. Synthesis and Evaluation of Troponoids as a New Class of Antibiotics. ACS Omega. 2018;3(11):15125-15133. doi:10.1021/acsomega.8b01754
22. Kodama T, Saito K, Tobisu M. Nickel-catalyzed skeletal transformation of tropone derivatives via C–C bond activation: catalyst-controlled access to diverse ring systems. Chem Sci. 2022;13(17):4922-4929. doi:10.1039/d2sc01394k
23. Liu S, He C, Liao Y, Liu H, Mao W, Shen Z. Enhancing and Complementary Mechanisms of Synergistic Action of Acori tatarinowii Rhizoma and Codonopsis radix for Alzheimer’s Disease Based on Systems Pharmacology. Evidence-Based Complement Altern Med. 2020;2020(1):1-26. doi:10.1155/2020/6317230
24. Kim HK, Choi YH, Lee EN, Park JK, Kim SG, Park DJ, Kim BS, Lim Y, Yoon S. 5‐Hydroxymethylfurfural From Black Garlic Extract Prevents TNFα‐induced Monocytic Cell Adhesion to HUVECs by Suppression of Vascular Cell Adhesion Molecule‐1 Expression, Reactive Oxygen Species Generation and NF‐κB Activation. Phyther Res. 2011;25(7):965-974. doi:10.1002/ptr.3351
25. Wölkart G, Schrammel A, Koyani CN, Scherübel S, Zorn-Pauly K, Malle E, Pelzmann B, Andrä M, Ortner A, Mayer B. Cardioprotective effects of 5‐hydroxymethylfurfural mediated by inhibition of L‐type Ca2+ currents. Br J Pharmacol. 2017;174(20):3640-3653. doi:10.1111/bph.13967
26. MacLeod CM. Trehalose Enhances Mitochondria Deficits in Human NPC1 Mutant Fibroblasts but Disrupts Mouse Purkinje Cell Dendritic Growth Ex Vivo. PLoS One. 2023;18(11):e0294312. doi:10.1371/journal.pone.0294312
27. Evans TD, Jeong SJ, Zhang X, Sergin I, Razani B. TFEB and Trehalose Drive the Macrophage Autophagy-Lysosome System to Protect Against Atherosclerosis. Autophagy. 2018;14(4):724-726. doi:10.1080/15548627.2018.1434373
28. Sergin I, Evans TD, Zhang X, Bhattacharya S, Stokes CJ, Song E, Ali S, Dehestani B, Holloway KB, Micevych PS, Javaheri A, Crowley JR, Ballabio A, Schilling JD, Epelman S, Weihl CC, Diwan A, Fan D, Zayed MA, Razani B. Exploiting Macrophage Autophagy-Lysosomal Biogenesis as a Therapy for Atherosclerosis. Nat Commun. 2017;8(1):1-20. doi:10.1038/ncomms15750
29. Stevanovic D. Trehalose Attenuates in Vitro Neurotoxicity of 6-Hydroxydopamine by Reducing Oxidative Stress and Activation of MAPK/AMPK Signaling Pathways. Int J Mol Sci. 2024;25(19):10659. doi:10.3390/ijms251910659
30. Echigo R, Shimohata N, Karatsu K, Yano F, Kayasuga-Kariya Y, Fujisawa A, Ohto T, Kita Y, Nakamura M, Suzuki S, Mochizuki M, Shimizu T, Chung U, Sasaki N. Trehalose Treatment Suppresses Inflammation, Oxidative Stress, and Vasospasm Induced by Experimental Subarachnoid Hemorrhage. J Transl Med. 2012;10(1):1-13. doi:10.1186/1479-5876-10-80
31. Allavena G, Bello BD, Tini P, Volpi N, Valacchi G, Miracco C, Pirtoli L, Maellaro E. Trehalose Inhibits Cell Proliferation and Amplifies Long‐term Temozolomide‐ and Radiation‐induced Cytotoxicity in Melanoma Cells: A Role for Autophagy and Premature Senescence. J Cell Physiol. 2018;234(7):11708-11721. doi:10.1002/jcp.27838
32. Frapporti G, Colombo E, Ahmed H, Assoni G, Polito L, Randazzo P, Seneci P, Piccoli G. Squalene-Based Nano-Assemblies Improve the Pro-Autophagic Activity of Trehalose. Pharmaceutics. 2022;14(4):862:1-17. doi:10.3390/pharmaceutics14040862