Phytochemical Constituents of Justicia carnea Leaves and their Antibacterial Activity
Main Article Content
Abstract
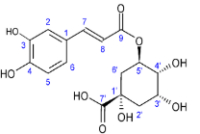
The use of plant-based therapeutics in the treatment of bacterial infections is of utmost importance in ethnomedicine and the research into the bioactive constituents of ethnomedicinal plants is still ongoing. Hence, this research aimed to determine the phytochemical constituents of Justicia carnea extracts and their antibacterial activities against Streptococcus pneumoniae, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, and Salmonella typhi, which are responsible for infections such as urinary tract infection, sepsis, abscesses, pneumonia and diarrhea. The methanol (MCE), n-hexane (HCE), chloroform (CCE), and methanol/water (MWCE) crude extracts were prepared using the pulverized leaves of J. carnea. The crude extract was extracted using the column chromatographic technique while purification was achieved using Sephadex LH-20 over flash chromatography. The isolated compounds’ purity was assessed using TLC and melting point. 1D and 2D NMR spectra identified chlorogenic acid (1), quinaldic acid (2), and oleic acid (3) as compounds in n-hexane leaves extract. We report for the first time the isolation of quinaldic and chlorogenic acids from J. carnea. The antibacterial activities were assessed based on the average diameter of the zone of inhibition, MIC, and MBC. All bacterial assayed were susceptible to MCE (≥ 11.2 ± 0.12 mm), HCE (≥ 13.1 ± 0.09 mm), CCE (≥ 9.2 ± 0.12 mm), MWCE (≥ 10.10 ± 0.10 mm), chlorogenic acid (1) (≥ 11.1 ± 0.13 mm), quinaldic acid (2) (≥ 8.1 ± 0.07 mm) and not to oleic acid (3) (≤ 2.1 ± 0.07 mm). The results showed that J. carnea contained medicinal compounds that are viable antibacterial agents.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Corrêa GM, Alcântara AC. Chemical constituents and biological activities of species of Justicia - A review. Rev. Bras. Farmacogn. 2011; 1-19.
Anigboro AA, Avwioroko OJ, Ohwokevwo OA, Pessu B, Tonukari NJ. Phytochemical Profile, antioxidant, α-amylase, inhibition, binding interaction and docking studies of Justicia carnea bioactive compounds with α-amylase. Biophys. Chem. 2021; 269: 106529.
Nirmalraj S, Ravikumar M, Mahendrakumar M, Bharath B, Perinbam K. Antibacterial and anti-inflammatory activity of Justicia gendarussa Burm. F. Leaves. J. Plant Sci. 2015; 10: 70-74.
Anarado CE, Ajiwe VIE, Anarado CJO, Obumselu OF, Umedum NL, Okafor SE. The phytochemistry, ethnomedicinal and pharmacology uses of Justicia carnea Lindl used in traditional medicine in Nigeria - A review. South Asian Res. J. Nat. Prod. 2021; 4(4): 85-93.
Ijoma KI, Ajiwe VIE. Jatropha tanjorensis a flora of southeast Nigeria: isolation and characterization of naringenin and validation of bio-enhanced synergistical activity of α- tocopherol toward clinical isolates of resistant bacterial. Mak. J. Sci. 2022; 26(2):120-127.
Wianowska D, Gil M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 2019; 18(1): 273-302.
Znati M, Jannet HB, Cazaux S, Souchard JP, Skhiri HF, Bouajila J. Antioxidant, 5-lipoxygenase inhibitory and cytotoxic activities of compounds isolated from the ferula lutea flowers. Molecules. 2014; 19(10): 16959-16975.
Kaslow CE, Stayner DR. Ozonolysis of styryl derivatives of nitrogen heterocycles. J. Am. Chem. Soc. 1945; 67(10): 1716-1717.
Starratt AN, Caveney S. Quinoline-2-carboxylic acid from Ephedra species. Phytochemistry. 1996; 42(5): 1477- 1478.
Lee C, Lee H. Growth inhibiting activity of quinaldic acid isolated from Ephedra pachyclada against intestinal bacteria. J. Korean Soc. Appl. Biol. Chem. 2009; 52(4): 331-335.
Japir A, Salimon J, Derawi D, Yahaya BH, Bahadi M, Al-shuja’a S, Yusop MR. A highly efficient separation and physicochemical characteristics of saturated fatty acids from crude palm oil fatty acids mixture using methanol crystallization method. Oilseeds and Fats Crops and Lipids 2018; 25(2): A203.
Malarvizhi D, Anusooriya P, Meenakshi SS, Oirere E, Gopalakrishnan VK. Isolation, structural characterization of oleic acid from Zaleya decandra root extract. Anal. Chem. Lett. 2016; http://dx.doi.org/10.1080/22297928.2016.1238319.
Oladapo AS, Akinyosoye FA, Abiodun OA. The inhibitory effect of different chemical food preservatives on the growth of selected food borne pathogenic bacteria. Afr. J. Microbiol. Res. 2014; 8(14), 1510-1515.
Ramli H, Baharudin Z, Hanafiah RM. (2022). Antibacterial activity of bioactive compounds in Salvadora Persia (chewing sticks) against Porphyromona gingivalis and Aggregatibacter actinomycetemcomitans. Akademi Sains Malay. Sci. J. 2022; 17: 1-8.
Riski DG, Maulana RGR, Permana E, Lestari I, Tarigan IL. Profile analysis of fatty acids of tengkawang (Shorea Sumatrana) oil using GC-MS and antibacterial activity. Indonesian J. Chem. Res. 2020; 8(2): 114-119.
Teh CH, Nazni WA, Nurulhusna AH, Ahmad NA, Lee HL. Determination of antibacterial activity and minimum inhibitory concentration of larval extract of fly via resazurin-based turbidometric assay. BMC Microbiol. 2017; 17(36): 1-8.
Jumina J, Lavendi W, Singgih T, Triono S, Kurniawan YS, Koketsu M. Preparation of monoacylglycerol from Indonesian edible oil and their antibacterial activity against Staphylococcus aureus and Escherichia coli. Sci. Rep. 2019; 9(10941): 1-8.
Dilika F, Bremner PD, Meyer JJM. Antibacterial activity of linoleic acid and oleic acid isolated from Helichrysum pedunculatum: a plant used during circumcision. Fitoterapia. 2000; 17: 450-452.
Mujtaba A, Masud T, Ahmad A, Ahmed W, Jabbar S, Levin RE. Antibacterial activity by chlorogenic acid isolated through resin from apricot (Prunus Armeniaca L.). Pak. J. Agric. Res. 2017; 30(2): 144-148.
Zhai T, Haider S, Liu Y, Huang ZJ. Synergistic effect of chlorogenic acid and caffeic acid with Fosfomycin on growth inhibition of a resistant Listeria monocytogenes strains. ACS Omega. 2020; 5(13): 7537-7544
Sakamoto F, Ikeda S, Tsukamoto G. Quinoline carboxylic acid derivative, process for production thereof, and antibacterial agent containing said compound as active ingredient. European patent application no. 0067411A1, 1-35, 1982.
Arunkumar K, Renjan T, van Belkum A, Vasanthakumari N. In vitro antibacterial and antibiofilm activities of chlorogenic acid against clinical isolates of Stenotrophomonas maltophilia including the trimethoprim/sulfamethoxazole resistant strain. Bio. Res. Intern. 2013; 392058: 1-7.
Neetu N, Katiki M, Dev A, Gaur S, Tomar S, Kumar P. Structural and biochemical analysis reveal chlorogenic acid inhibits the shikimate pathway. J. bacteriol. 2020; 202(18): e00248-20.
Chai B, Jiang W, Hu M, Wu Y, Si H. In vitro synergistic interactions of protocatechuic acid and chlorogenic acid in combination with antibiotics against animal pathogens. Synergy. 2019; 9: 6-11.
Ijoma KI, Okafor CE, Ajiwe VIE. Computational Studies of 5- methoxypsolaren as Potential Deoxyhemoglobin S Polymerization Inhibitor. Trop J Nat Prod Res. 2024; 8(10): 8835 – 8841 https://doi.org/10.26538/tjnpr/v8i10.28