Antibacterial and Antibiofilm Properties of Jicama (Pachyrhizus erosus) Seed Extract against Streptococcus mutans: Identification of Key Bioactive Compounds Using Bioautography and LC-MS/MS Analysis
Main Article Content
Abstract
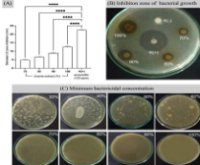
Streptococcus mutans (S. mutans) is the primary bacterium responsible for dental caries, forming biofilms that enhance resistance to antimicrobial treatments. This study aimed to evaluate the antibacterial and antibiofilm activities of jicama seed extract (Pachyrhizus erosus) against S. mutans, as well as to identify the bioactive compounds responsible for these effects. Jicama seeds were extracted using a 70% ethanol solution, and antibacterial activity was assessed using the disc diffusion method, while antibiofilm properties were analyzed via microplate assays. Thin-layer chromatography and bioautography were conducted to identify active compounds further characterized by LC-MS/MS analysis. The results indicated that a 70% concentration of jicama extract inhibited bacterial growth, with inhibition zones increasing proportionally with extract concentrations. Bioautographic and LC-MS/MS analysis confirmed the presence of antibacterial compounds such as alkaloids and flavonoids, including hypaphorine, trigonelline, glabridin, and gancaonin. This compound significantly inhibited S. mutans biofilm formation. The LC-MS/MS results also revealed various bioactive compounds with potential antibacterial properties. These findings suggest that jicama seed extract could serve as a natural antibacterial agent, with potential applications in dental care. Further studies should explore optimal extraction methods to enhance its effectiveness and safety in clinical use.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Banas JA, Vickerman MM. Glucan-binding protein of the oral Streptococci. Crit Rev Oral Biol Med. 2003; 14(2):89–99.
2. Zayed SM, Aboulwafa MM, Hashem AM, Saleh SE. Biofilm formation by Streptococcus mutans and its inhibition by green tea extracts. AMB Express [Internet]. 2021; 11(1):73.Available from: https://doi.org/10.1186/s13568-021-01232-6.
3. Nonong YH, Satari MH, Indriyanti R, Patawulandari S. Antibacterial test between Aloe vera and chlorhexidine based on the number of colony of Streptococcus mutans ATCC 25 175 in vitro. Int J Sci Res. 2016; 5(1):1379–1385.
4. Sedarat Z, Taylor-Robinson AW. Biofilm formation by pathogenic bacteria: Applying a Staphylococcus aureus model to appraise potential targets for therapeutic intervention. Pathogens. 2022; 11(4):388.
5. Tolker-Nielsen T. Biofilm development. Microbiol Spectrum. 2015; 3(2): 1-12
6. Belattar N, Barhouchi B, Benayache S, Merabet S. Insights on phytochemicals as antimicrobial potentiators : a review. J Mol Pharm Sci. 2023; 2(02):59-109.
7. Rahminiwati M, Ramadhan J, Komala O. Antimicrobial activity of 70% ethanol extract of jicama seeds against Staphylococcus epidermidis, Pseudomonas aeruginosa and Candida albican. JSV. 2020; 38(3):289-298.
8. Juriah J. Fractionation of jicama seed extract (Pachyrrhizus erozus) which has the potential to be antibacterial. Bogor Agricultural University (IPB); 2003.
9. AOAC International. Official methods of analysis of AOAC International. AOAC international; 2000.
10. Tortora GJ, Funke BR, Case CL. Microbiology: an introduction. 10 th. Pearson Benjamin Cummings, San Francisco; 2010. p. 572.
11. Pratiwi. Pharmaceutical microbiology. Jakarta. Erlangga; 2008. p.188-191.
12. Aslah A, Lolo WA, Jayanto I. Antibacterial activity and TLC-bioautographic analysis of noni leaf fractions (Morinda citrifolia L.). Pharmacon. 2019; 8(2):505-515.
13. Mustiqawati E, Yolandari S. Identification of saponin compounds in lime leaf extract (Citrus aurantifolia S.) by Thin Layer Chromatography. JPP. 2022; 5(2):66–73.
14. Izzah N, Kadang Y, Permatasari A. Identification test of alkaloid compounds of moringa leaf methanol extract (Moringa oleifera Lamk) from Ende Regency, East Nusa Tenggara by thin layer chromatography. J Farm Sandi Karsa. 2019; 5(1):52-56.
15. Kusumaningtyas E, Astuti E, Darmono D. Sensitivity of contact bioautography and agar overlay methods in determining anti-mold compounds. JIFI. 2008, 6(2) : 75-80.
16. Baderos, A. 2017. Separation of petroleum ether fractional steroid compounds of red algae (Eucheuma cottonii) by thin-layer chromatography method and identification using LC-MS. Thesis. Faculty of Science and Technology, Maulana Malik Ibrahim State Islamic University. 2017.
17. Tobi CHB, Saptarini O, Rahmawati I. Antibiofilm activity of areca nut (Areca catechu L) extract and fractions against Staphylococcus aureus ATCC 25923. JPSCR. 2022; 1:56-70
18. Wulandari L. Thin-layer chromatography. Jember: PT. Taman Kampus Presindo.; 2011.
19. Supari H, Leman MA, Zuliari K. Antibacterial effectiveness of jicama seed extract (Pachyrrhizus erosus) on the growth of Streptococcus mutans in vitro. Pharmacon. 2016; 5(3):33–39.
20. Martins ML, de França Leite KL, Pacheco-Filho EF, de Miranda Pereira AF, Romanos MT, Maia LC, Fonseca-Gonçalves A, Padilha WW, Cavalcanti YW. Efficacy of red propolis hydro-alcoholic extract in controlling Streptococcus mutans biofilm build-up and dental enamel demineralization. Arch Oral Biol [Internet]. 2018; 93:56–65. Available from: https://doi.org/10.1016/j.archoralbio.2018.05.017.
21. Araghizadeh A, Kohanteb J, Fani MM. Inhibitory activity of green tea (Camellia sinensis) extract on some clinically isolated cariogenic and periodontopathic bacteria. Med Princ Pract. 2013; 22(4):368–372.
22. Halimah, Maryani Y, Krisyudhanti E, Wardoyo S. Effectiveness of secang wood (Caesalpinia sappan L.) in inhibiting the growth of Streptococcus mutans. Trop J Nat Prod Res (TJNPR). 2024; 8(6):7504–7508.
23. Hardoko H, Sipayung JK, Halim Y. Antibacterial activity of Rhizophora mucronata leaves extract and its application in chewing gum against Streptococcus mutans and Streptococcus viridans: . Trop J Nat Prod Res (TJNPR). 2021 Dec 1; 5(12):2068-2073. doi. org/10.26538/tjnpr/v5i12.
24. Ifemeje JC, Ifemeje MO, Egbuna C, Olisah MC. Proximate, phytochemical and antioxidant mineral compositions of four different brands of tea. Adv J Grad Res. 2020; 8(1):1–7.
25. Yan Y, Li X, Zhang C, Lv L, Gao B, Li M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics. 2021; 10(3):318. .https://doi.org/10.3390/antibiotics10030318.
26. Princy KR, Sripathi R, Dharani J, Ravi S. Molecular docking studies of alkaloids from Desmodium triflorum against bacterial proteins. J Pharm Sci Res. 2017; 9(10):1882–1885.
27. Talla E, Fotsing MC, Ismaila MB, Tata CM, Ikhile MI, Rhyman L, Arderne C, Niemann N, Ramasami P, Ndinteh DT. Density functional theory studies of hypaphorine from Erythrina mildbraedii and Erythrina addisoniae: Structural and biological properties. SN Appl Sci [Internet]. 2020; 2(3):1–12. Available from: https://doi.org/10.1007/s42452-42020-2228-z.
28. Antonio AG, Moraes RS, Perrone D, Maia LC, Santos KR, Iório NL, Farah A. Species, roasting degree and decaffeination influence the antibacterial activity of coffee against Streptococcus mutans. Food Chem [Internet]. 2010;118(3): 782–788. Available from: http://dx.doi.org/10.1016/j.foodchem.2009.05.063.
29. Kar A, Mukherjee SK, Barik, S. and Hossain ST, Hossain ST. Antimicrobial activity of trigonelline hydrochloride against Pseudomonas aeruginosa and its quorum-sensing regulated molecular mechanisms on biofilm formation and virulence. ACS Infect Dis. 2024;10(2): 746–762.
30. Cvitkovitch DG, Senadheera D. Quorum sensing and biofilm formation by Streptococcus. Adv Exp Med Biol. 2008:631: 178–188.
31. Kaur G, Rajesh S, Princy SA. Plausible drug targets in the Streptococcus mutans quorum sensing pathways to combat dental biofilms and associated risks. Indian J Microbiol. 2015;55(4): 349356.
32. Muras A, Mayer C, Romero M, Camino T, Ferrer MD, Mira A, Otero A. Inhibition of Steptococcus mutans biofilm formation by extracts of Tenacibaculum sp. 20J, a bacterium with wide-spectrum quorum quenching activity. J Oral Microbiol. 2018; 10(1):1429788. 2018, VOL. 10, 1429788https://doi.org/10.1080/20002297.2018.1429788.
33. Araya-Cloutier C, Vincken JP, van de Schans MG, Hageman J, Schaftenaar G, den Besten HM, Gruppen H. QSAR-based molecular signatures of prenylated (iso)flavonoids underlying antimicrobial potency against and membrane-disruption in Gram positive and Gram negative bacteria. Sci Rep. 2018; 8(1):1–15.
34. Vaillancourt K, Lebel G, Pellerin G, Lagha A Ben, Grenier D. Effects of the licorice isoflavans licoricidin and glabridin on the growth, adherence properties, and acid production of Streptococcus mutans, and assessment of their biocompatibility. Antibiotics. 2021; 10(2):1–10.
35. He J, Chen L, Heber D, Shi W, Lu QY. Antibacterial compounds from Glycyrrhiza uralensis. J Nat Prod. 2006; 69(1):121–124.
36. Gupta VK, Fatima A, Faridi U, Negi AS, Shanker K, Kumar JK, Rahuja N, Luqman S, Sisodia BS, Saikia D, Darokar MP. Antimicrobial potential of Glycyrrhiza glabra roots. J Ethnopharmacol. 2008; 116(2):377–380.
37. Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976; 40(3):681–697.
38. Refai MY, Elazzazy AM, Desouky SE, Abu-Elghait M, Fayed EA, Alajel SM, Alajlan AA, Albureikan MO, Nakayama J. Interception of epoxide ring to quorum sensing system in Enterococcus faecalis and Staphylococcus aureus. AMB Express. 2023;13(1):126.
39. Casella TM, Eparvier V, Mandavid H, Bendelac A, Odonne G, Dayan L, Duplais C, Espindola LS, Stien D. Antimicrobial and cytotoxic secondary metabolites from tropical leaf endophytes: Isolation of antibacterial agent pyrrocidine C from Lewia infectoria SNB-GTC2402. Phytochemistry [Internet]. 2013; 96(2013):370–377. Available from: http://dx.doi.org/10.1016/j.phytochem.2013.10.004.
40. Phuong NT, Van Quang N, Mai TT, Anh NV, Kuhakarn C, Reutrakul V, Bolhuis A. Antibiofilm activity of α-mangostin extracted from Garcinia mangostana L. against Staphylococcus aureus. Asian Pac J Trop Med [Internet]. 2017; 10(12):1154–1160. Available from: https://doi.org/10.1016/j.apjtm.2017.10.022.
41. Zhao DL, Wang D, Tian XY, Cao F, Li YQ, Zhang CS. Anti-phytopathogenic and cytotoxic activities of crude extracts and secondary metabolites of marine-derived fungi. Mar Drugs. 2018; 16(1):1–15.
42. Lin S, Zhu C, Li H, Chen Y, Liu S. Potent in vitro and in vivo antimicrobial activity of semisynthetic amphiphilic γ-mangostin derivative LS02 against Gram-positive bacteria with destructive effect on bacterial membrane. BBA - Biomembrans [Internet]. 2020; 1862(9):183353. Available from: https://doi.org/10.1016/j.bbamem.2020.183353.
43. Lautié E, Rozet E, Hubert P, Quetin Leclercq J. Quantification of rotenone in seeds of different species of yam bean (Pachyrhizus sp.) by a SPE HPLC-UV method. Food Chem. 2012;131(4): 1531–1538.
44. Catteau L, Lautié E, Koné O, Coppée M, Hell K, Pomalegni CB, Quetin-Leclercq J. Degradation of rotenone in yam bean seeds (Pachyrhizus sp.) through food processing. J Agric Food Chem. 2013; 61(46): 11173–11179.
45. Obatolu VA, Fasoyiro SB, Ogunsunmi L. Processing and functional properties of yam beans (Sphenostylis stenocarpa). J Food Process Preserv. 2007; 31(2):240–249.
46. Xiao XN, Wang F, Yuan YT, Liu J, Liu YZ, Yi X. Antibacterial activity and mode of action of dihydromyricetin from Ampelopsis grossedentata leaves against food-borne bacteria. Molecules. 2019; 24(15):2831.
47. Çitoǧlu GS, Sever B, Antus S, Baitz-Gács E, Altanlar N. Antifungal diterpenoids and flavonoids from Ballota inaequidens. Pharm Biol. 2004; 42(8):659–663.
48. Santhosh Kumar Y, Kulanthaivel L, Hikku GS, Saravanan R, Lakshmi T, Kirubhanand C, Karthikeyan M, Vijayalakshmi P, Subbaraj GK. Improved antibacterial activity of water-soluble nanoformulated kaempferol and combretastatin polyphenolic compounds. Int J Polym Sci. 2021; 2021(1):5682182. https://doi.org/10.1155/2021/5682182.
49. Lopes LAA, dos Santos Rodrigues JB, Magnani M, de Souza EL, de Siqueira-Júnior JP. Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that overexpresses efflux protein genes. Microb Pathog. 2017; 107:193–197.
50. Pinto HB, Brust FR, Macedo AJ, Trentin DS. The antivirulence compound myricetin possesses remarkable synergistic effect with antibacterials upon multidrug resistant Staphylococcus aureus. Microb Pathog. 2020; 149:104571.