Optimization of Extraction Condition and the Antioxidant Activity of Momordica charantia Leaves from Vietnam
Main Article Content
Abstract
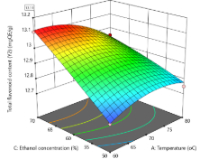
The growing interest in natural bioactive compounds has emphasized the need for efficient extraction techniques to maximize the yield of key phytochemicals. This study explores the ideal parameters, including extraction temperature, solvent concentration, and extraction duration, to optimize the extraction process and achieve maximum yields of total flavonoid content (TFC) and total phenolic content (TPC) from Momordica charantia leaves. Utilizing response surface methodology (RSM), the extraction process was optimized, revealing that the polynomial expressions for every model demonstrated significance in the obtained results. The result shows that the TPC (87.85 ± 0.24 mgGAE/g DW) and TFC (12.68 ± 0.17 mgQE/g DW) could be extracted higher in the optimum operating conditions at 72°C for 70 min and 70% ethanol concentration.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Lucas EA, Arjmandim BH. Health Benefits of Bitter Melon (Momordica charantia). Bioactive Foods in Promoting Health. 2010; 525-549. https://doi.org/10.1016/B978-0-12-374628-3.00035-9
Batran SA, Gengaihi SE, Shabrawy OA. Some toxicological studies of Momordica charantia L. on albino rats in normal and alloxan diabetic rats. J. Ethnopharmacol. 2006; 108: 236-242. https://doi.org/10.1016/j.jep.2006.05.015
Grover JK, Yadav SP. (2004). Pharmacological actions and potential uses of Momordica charantia: a review. J. Ethnopharmacol. 2004; 93(1): 123-132. https://doi.org/10.1016/j.jep.2004.03.035
Gürbüz I, Akyüz C, Yesilada E, Sener B. Anti-ulcerogenic effect of Momordica charantia L. fruits on various ulcer models in rats. J. Ethnopharmacol. 2000; 71(1-2): 77-82. https://doi.org/10.1016/S0378-8741(99)00178-6
Subair KB, Ade-Ademilua EO, Osinubi AA. Effect of Momordica charantia Leaf Extract on Female Fertility and Foetal Well-Being in Sprague-Dawley Rats. Trop J Nat Prod Res. 2022; 6(10): 1719-1722. http://www.doi.org/10.26538/tjnpr/v6i10.27
Lee HS, Huang PL, Chen HC, Huang PL, Bourinbaiar A, Huang HI, Kung HF. Anti-HIV and anti-tumor activities of recombinant MAP30 from bitter melon. Gene. 1995; 161(2): 151-156. https://doi.org/10.1016/0378-1119(95)00186-a
Guevara AP, Lim-Sylianco C, Dayrit F, Finch P. Antimutagens from Momordica charantia. Mutat. Res. 1990; 230(2): 121-126, https://doi.org/10.1016/0027-5107(90)90050-E
Spreafico F, Malfiore C, Moras ML, Marmonti L, Filippeschi S, Barbieri L, Perocco P, Stirpe F. The immunomodulatory activity of the plant proteins Momordica charantia inhibitor and pokeweed antiviral protein. Int. J. Immunopharmacol. 1983; 5(4): 335-343. https://doi.org/10.1016/0192-0561(83)90037-1
Prasesti GK, Anggadiredja K, Kurniati NF. Momordica charantia Fruit Extract on Cardiac Biomarker Serum Attenuation in Rats and its Bioactive Compound Molecular Docking Against SIRT-1Protein. Trop J Nat Prod Res. 2023; 7(1): 2229-2233. http://www.doi.org/10.26538/tjnpr/v7i1.21
Singh A, Singh PS, Bamezai R. Momordica charantia L. (Bitter Gourd) peel, pulp, seed and whole fruit extract inhibits mouse skin papillomagenesis. Toxicol Letters. 1998; 94(1): 37- 46. https://doi.org/10.1016/s0378-4274(97)00099-4
Patil SA, Patil SB. Toxicological studies of Momordica charantia Linn Seed extracts in Male Mice. International Journal of Morphology. 2011; 29(4): 1212-1218. http://dx.doi.org/10.4067/S0717-95022011000400024
Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology. 1999; 299: 152-178. https://doi.org/10.1016/S0076-6879(99)99017-1
Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoids content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis. 2022; 10(3): 178-82. https://doi.org/10.38212/2224-6614.2748
Ijoma KI. The organic extracts from the leaves of Ficus thonningii Blume, Jatropha tanjorensis J.L Ellis and Saroja and Justicia carnea Lindley as potential nutraceutical antioxidants and functional foods. Trends Phytochem. Res. 2023 ; 7(1) : 76-85 https://doi.org/10.30495/tpr.2023.1977670.1318
Biglari F, AlKarkhi AFM, Easa AM. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits for Iran. Food Chem. 2008; 107(4): 1636–1641. https://doi.org/10.1016/j.foodchem.2007.10.033
Lo KM, Cheung PCK. (2005). Antioxidant activity of extracts from the fruiting bodies of Agrocybe aegerita var. alba. Food Chem. 2005; 89(4): 533–539, https://doi.org/10.1016/j.foodchem.2004.03.006
Yilmaz Y, Toledo RT. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. Journal of Food Composition and Analysis. 2006; 19(1): 41–48. https://doi.org/10.1016/j.jfca.2004.10.009
Chi TP, Trang BT, Trinh NTN, Tho LTC, Thanh NT, Thang TD, Hang HTL, Quang LD, Tuan NN. Optimization of Ultrasonic-Assisted Extraction of Antioxidant Compounds in Black Shallots (Allium ascalonicum) from Vietnam using Response Surface Methodology. Malaysian Journal of Chemistry. 2022; 24(1): 26-35
Vajić UJ, Grujić-Milanović J, Živković J, Šavikin K, Gođevac D, Miloradović Z, Bugarski B, Mihailović-Stanojević N. Optimization of extraction of stinging nettle leaf phenolic compounds using response surface methodology. Industrial Crops and Products. 2015; 74: 912–917. https://doi.org/10.1016/j.indcrop.2015.06.032
Tan MC, Tan CP, Ho CW. Effects of extraction solvent system, time and temperature on total phenolic content of henna (Lawsonia inermis) stems. Int. Food Res. J. 2013; 20(6): 3117–3123
Gomes T, Delgado T, Ferreira A, Pereira JA, Baptista P, Casal S, Ramalhosa E. Application of response surface methodology for obtaining lettuce (Lactuca sativa L.) by-products extracts with high antioxidative properties. Industrial Crops and Products. 2013; 44: 622–629. https://doi.org/10.1016/j.indcrop.2012.09.011
Lu J, Zhou C, Rong O, Xu Y. Optimization of microwave-assisted extraction of flavonoids from Cryptotaenia japonica hassk using response surface methodology. Adv. J. Food Sci. Technol.. 2013; 5(3): 310–317. https://doi.org/10.19026/ajfst.5.3262
Thanh NT. Optimization of Microwave-Assisted Extraction of Antioxidant Compounds from Roots of Polygonum multiflorum Thunb. At Vietnam using Response Surface Methodology. Malays. J. Chem.. 2023; 25(4), 21-29.