Structural Analysis of Saponin Isolate from the Soapbark Tree Extract
Main Article Content
Abstract
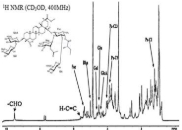
The outbreaks of pandemics and epidemics have reenacted interest in high molecular weight bioactive molecules. Saponin is one of these molecules because of its associated health and commercial benefits. In this perspective, saponins from the aqueous extract of the soapbark (Q. saponoria) tree are attracting a rejuvenated interest among scholars. Therefore, this study was to provide further structural clarity to one of the identified saponin constituents of the soapbark tree extract. Adopting standard procedures for gas-liquid chromatography and in hyphenation with mass spectrometry, the alditol acetates and the associated methylated derivatives of the saccharide linkages of the saponin were evaluated. The space tandem mass spectrometric analyses and the NMR evaluation of the saponin fraction were also carried out. Obtained data showed the presence of a carbonyl proton peak at C-23. The finding suggests a deviation from previous reports for similar saponins with phytolaccinic acid aglycone. And may explain the possible bioactivity benefit of the molecule.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Barber SL, Ong P, Nozaki S. [Special Article] The 40th anniversary of the Declaration of Alma Ata. Universal health coverage and the renewal of primary health care: a commentary. J Int Health, 2018; 33(2): 93–97.
Briggs AM, Shiffman J, Shawar YR, Åkesson K, Ali N, Woolf AD. Global health policy in the 21st century: Challenges and opportunities to arrest the global disability burden from musculoskeletal health conditions. Best Pract Res Clin Rheumatol., 2020; 34(5):101549; https://doi.org/10.1016/j.berh.2020.101549.
World Health Organization (WHO), Regional Office for Europe. Facing the future: opportunities and challenges for 21st-century public health in implementing the Sustainable Development Goals and the Health 2020 policy framework. WHO, 2018; Available [online]: https://iris.who.int/handle/10665/340350; Accessed Date: 4th March 2024.
Park S, Abrams R. Alma-Ata 40th birthday celebrations and the Astana Declaration on Primary Health Care 2018. Br. J. Gen. Pract., 2019; 69(682):220-221. http://doi.org/10.3399/bjgp19X702293.
Kumar P, Ekka P. Statistical analysis of the impacts of COVID-19 pandemic on the small and large-scale tourism sectors in developing countries. Environ Dev Sustain., 2023; https://doi.org/10.1007/s10668-023-03112-4.
Bommakanti V, Puthenparambil AA, Sivi CM, Prakash G, Mundanat AS, Ahmad F, Haque S, Prieto MA, Rana SS. An Overview of herbal nutraceuticals, their extraction, formulation, therapeutic effects and potential toxicity. Separations 2023; 10(3):177; https://doi.org/10.3390/separations10030177.
Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol., 2014; 10;4:177; https://doi:10.3389/fphar.2013.00177.
Tebogo MO, Monkgogi T, Moshapa F, Rapaka D, Bitra VR, Adiukwu PC. Liquid chromatography-electrospray ionization mass spectrometry analysis of Quil-A: an aqueous extract from the bark of Quillaja saponaria Molina. Trop J Nat Prod Res., 2023; 7(1):2285-2291; http://www.doi.org/10.26538/tjnpr/v7i1.30.
Fleck DJ, Betti AH, da Silva FP, Troian EA, Olivaro C, Ferreira F, Verza SG. Saponins from Quillaja saponaria and Quillaja brasiliensis: particular chemical characteristics and biological activities. Molecules, 2019; 24(1):171; https://doi.org/10.3390/molecules24010171.
Reed J, Orme A, El-Demerdash A, Owen C, Martin Laetitia BB, Misra RC, Kikuchi S, Rejzek M, Azahara CM, Harkess A, Leebens-Mack J, Louveau T, Stephenson MJ, Osbourn A. Elucidation of the pathway for biosynthesis of saponin adjuvants from the soapbark tree. Science, 2023; 379 (6638):1252; https://doi.org/10.1126/science.adf3727.
Luebert, F. Taxonomy and distribution of the genus Quillaja molina (Quillajaceae). Feddes Repert, 2014; 124, 157–162; https://doi.org/10.1002/fedr.201400029.
Yanira M, Aldrin VV, Thomas E, Kai S, Mohammad Y, Mehdi DD, Ludger AW, Carlos AG, Daniel G. Rivera, Bernhard Westermann. Diversification of a novel α‐galactosyl ceramide hotspot boosts the adjuvant properties in parenteral and mucosal vaccines. Angew. Chem. Int. Ed., 2023; 2024;63:e2023109; https://doi.org/10.1002/anie.202310983
Elkaradawy A, Abdel-Rahim MM, Albalawi AE, Althobaiti NA, Abozeid AM, Mohamed RA. Synergistic effects of the soapbark tree, Quillaja saponaria and vitamin E on water quality, growth performance, blood health, gills and intestine histomorphology of Nile tilapia, Oreochromis niloticus fingerlings. Aquac. Rep., 2021; 20;2021:100733; https://doi.org/10.1016/j.aqrep.2021.100733.
Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 1991;146(2):431–437
Fleck DJ, Betti AH, da Silva FP, Troian EA, Olivaro C, Ferreira F & Verza SG. Saponins from Quillaja saponaria and Quillaja brasiliensis: particular chemical characteristics and biological activities. Molecules, 2019; 24(1):171; https://doi.org/10.3390/molecules24010171.
Adiukwu CP, Tweteise UP, Tebogo MO, Deepthi R, Moshapa F, Veera RB. Semi-preparative RP-HPLC isolation and the pro-inflammatory, cytotoxicity, and erythrolytic study of saponin from the soapbark tree. Manuscript BMS-CBC-2024-133, submitted for publication.
Banoub JH, Michon F, Hodder HJ. Structural elucidation of the O-specific polysaccharide of the phenol-phase soluble lipopolysaccharide of Vibrio anguillarum. Biochem. Cell Biol., 1987; 65(1):19-26; https://doi.org/10.1139/o87-003.
Melton LD, Smith BG. Determination of Neutral Sugars by Gas Chromatography of their Alditol Acetates. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF,
Smith D, Sporns P., eds. Current Protocols in Food Analytical Chemistry, 2001; 00:E3.2.1-E3.2.13; https://doi.org/10.1002/0471142913.fae0302s00.
Hakomori SA. Rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J. Biochem., 1964; 55:205-208.
Amelung W, Cheshire MV, Guggenberger G. Determination of neutral and acidic sugars in soil by capillary gas–liquid chromatography after trifluoroacetic acid hydrolysis. Soil Biol Bioch., 1996; 28, 1631–1639.
Adams GA. Complete acid hydrolysis. In: Whistler RL, ed., Methods in Carbohydrate Chemistry 1965; Vol. 5: 269-276. Academic Press, New York.
Björndal H, Lindberg B, Pilotti A, Svensson S. Mass spectra of partially methylated alditol acetates: Part II. Deuterium Labelling Experiments. Carbohydr. Res., 1970; 15 (3):339-349; https://doi.org/10.1016/S0008-6215(00)80450-4.
Gerwig GJ. Analysis of carbohydrates by mass spectrometry. In: Kalyuzhny AE, Ed., The art of carbohydrate analysis. Techniques in life science and biomedicine for the non-expert. Springer, Cham 2021; https://doi.org/10.1007/978-3-030-77791-3_11.
Sloneker JH. Gas–liquid chromatography of alditol acetates. In: Whistler RL, BeMiller JN Eds., General carbohydrate method. Academic Press, 1972; Pages 20-24; https://doi.org/10.1016/B978-0-12-746206-6.50011-4.
Rumpel C, Dignac M-F. Gas chromatographic analysis of monosaccharides in a forest soil profile: Analysis by gas chromatography after trifluoroacetic acid hydrolysis and reduction–acetylation. Soil Biol. Biochem., 2006; 38:1478–1481.
Wallacea F, Bennadjib Z, Ferreira F, Olivaroa C. Analysis of an immunoadjuvant saponin fraction from Quillaja brasiliensis leaves by electrospray ionization ion trap multiple-stage mass spectrometry. Phytochem. Lett., 2017; 20:228-233; https://doi.org/10.1016/j.phytol.2017.04.020.
Brunton NP, Gormley TR, Murray B. Analytical Nutritional and Clinical Methods Use of the alditol acetate derivatization for the analysis of reducing sugars in potato tubers. Food Chem., 2007; 104:398–402; https://doi.org/10.1016/j.foodchem.2007.01.045.
Davison PK, Young R. Gas chromatography of carbohydrates, the quantitative determination of the free sugars of plants as their trimethylsilyl ethers. J. Chromatogr. A., 1967; 41:12–21; https://doi.org/10.1016/0021-9673(64)80093-5.
Su Y, Xia S, Wang R, Xiao L. Phytohormonal quantification based on biological principles. In: Li J, Li C, Smith SM, Eds., Hormone metabolism and signaling in plants. Academic Press, 2017; 431-470; https://doi.org/10.1016/B978-0-12-811562-6.00013-X.
Price NP. Permethylation linkage analysis techniques for residual carbohydrates. Appl Biochem Biotechnol., 2008; 148(1-3):271-276; https://doi.org/10.1007/s12010-007-8044-8.
Johnson A, Shimizu Y. Phytolaccinic acid, a new triterpene from phytolacca americana. Tetrahedron, 1974; 30:2033-2036;
Guo S, Kenne L. Structural studies of triterpenoid saponins with new acyl components from Quillaja saponaria Molina. Phytochemistry, 2000; 55:419-428; https://doi.org/10.1016/S0031-9422(00)00340-X.
Wallace F, Bennadji Z, Ferreira F, Olivaro C. Analysis of an immunoadjuvant saponin fraction from Quillaja brasiliensis leaves by electrospray ionization ion trap multiple-stage mass spectrometry. Phytochem. Lett., 2017; 20:228-233; https://doi.org/10.1016/j.phytol.2017.04.020.
Nord LI, Kenne L. Novel acetylated triterpenoid saponin in a chromatographic fraction from Quillaia saponaria Molina. Carbohydr. Res., 2000; 329:77-89; https://doi.org/1016/s0008-6215(00)00248-2.
Wang Y, Lu X, Xu G. Development of a comprehensive two-dimensional hydrophilic interaction chromatography/quadrupole time-of-flight mass spectrometry system and its application in separation and identification of saponins from Quillaja saponaria. J. Chromatogr. A., 2008; 1181(1–2):51-59; https://doi.org/10.1016/j.chroma.2007.12.034.
Bankefors J, Nordy LI, Kenne L. Structural classification of acyl-substituted Quillaja saponins by electrospray ionization ion trap multiple-stage mass spectrometry in combination with multivariate analysis. Rapid Commun. Mass Spectrom., 2008; 22:3851–3860, https://doi.org/10.1002/rcm.3803.
Baker JR, Zyzak DV, Thorpe SR, Baynes JW. Chemistry of the fructosamine assay: D-glucosone is the product of oxidation of Amadori compounds. Clin. Chem., 1994; 40, 1950–1955; https://doi.org/10.1093/clinchem/40.10.1950.
Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochem., 1995; 34, 3702–3709; https://doi.org/10.1021/bi00011a027.
Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J., 1999; 344:109–116. https://doi.org/10.1042/bj3440109.
Martins SIF, Marcelis AT, van Boekel MAJ. Kinetic modelling of Amadori N-(1-deoxy-d-fructos-1-yl)-glycine degradation pathways. Part I. Reaction mechanism. Carbohydr. Res., 2003; 338:1651–1663; https://doi.org/10.1016/s0008-6215(03)00173-3.
Calvano CD, Cataldi TRI, Kögel JF, Monopoli A, Palmisano F, Sundermeyer J. Structural characterization of neutral saccharides by negative ion MALDI mass spectrometry using a superbasic proton sponge as deprotonating matrix. J. Am. Soc. Mass Spectrom., 2017; 28(8):1666-1675; https://doi.org/10.1007/s13361-017-1679-y.
Domon B, Costello CE. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J., 1988; 5:397–409; https://doi.org/10.1007/bf01049915.
Kuuranne T, Vahermo M, Leinonen A, Kostiainen R. Electrospray and atmospheric pressure chemical ionization tandem mass spectrometric behavior of eight anabolic steroid glucuronides. J. Am. Soc. Mass Spectrom., 2000; 11(8):722-730; https://doi.org/10.1016/S1044-0305(00)00135-5.
Wallace F, Fontana C, Ferreira F, Olivaro C (2022). Structure elucidation of triterpenoid saponins found in an immunoadjuvant preparation of Quillaja brasiliensis using Mass spectrometry and 1H and 13C NMR spectroscopy. Molecules, 27(8):2402; https://doi.org/10.3390/molecules27082402.
Jansson, PE, Kenne L, Widmalm G. Computer-assisted structural analysis of polysaccharides with an extended version of CASPER using 1 H and 13C NMR data. Carbohydr. Res., 1989; 188:169-191; https://doi.org/10.1016/0008-6215(89)84069-8.
Agrawal, PK, NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry, 1992; 31:3307-3330; https://doi.org/10.1016/0031-9422(92)83678-r.
Jacobsen NE, Fairbrother WJ, Kensil CR, Lim A, Wheeler DA, Powell MF. Structure of the saponin adjuvant QS-21 and its base-catalysed isomerization product by 1 H and natural abundance 13C NMR spectroscopy. Carbohydr. Res., 1996; 280, 1-14; https://doi.org/10.1016/0008-6215(95)00278-2.
Guo S, Kenne L, Lundgren LN, RoÈnnberg B, Sundquist BG. Triterpenoid saponins from Quillaja saponaria. Phytochemistry, 1998; 48(1):175-180; https://doi.org/10.1016/s0031-9422(97)00716-4
Nord LI, Kenne L. Separation and structural analysis of saponins in a bark extract from Quillaja saponaria Molina. Carbohydr. Res., 1999; 320(1-2)71-81; https://doi.org/10.1016/s0008-6215(99)00134-2.
Nyberg NT, Kenne L, RoÈnnberg B, Sundquist BG. Separation and structural analysis of some saponins from Quillaja saponaria Molina. Carbohydr. Res., 2000; 323(1-4), 8797; https://doi.org/10.1016/s0008-6215(99)00227-x.
Kang SS, Woo WS. Two new saponins from Phytolacca americana. Planta Med., 1987; 53(4)338-340; https://doi.org/10.1055/s-2006-962732.
Bandara BMR, Jayasinghe ULB, Karunaratne V, Wannigama GP, Bokel M, Sotheeswaran S. Triterpenoidal constituents of Diploclisia glaucescens. Planta Med., 1990; 56(3):290-292; https://doi.org/10.1055/s-2006-960960.
Rastrelli, L., De Simone, F., Schettino, O., Dini, A., 1996. Constituents of Chenopodium pallidicaule (Canihua) seeds: isolation and characterization of new triterpene saponins. J. Agric. Food Chem., 1996; 44(11):3528-3533; https://doi.org/10.1021/jf950253p.
Marty-Roix R, Vladimer GI, Pouliot K, Weng D, Buglione-Corbett R, West K, MacMicking JD, Chee JD, Wang S, Lu S, Lien E. Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J. Biol. Chem., 2016; 291(3):1123–1136; https://doi.org/10.1074/jbc.m115.683011.