The Potency of Adenostemma platyphyllum and A. madurense as Antioxidant and Anti-aging Agents
Main Article Content
Abstract
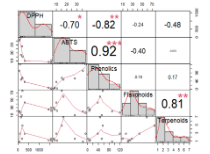
Adenostemma platyphyllum and Adenostemma madurense are medicinal plants belonging to the same genus as A. lavenia. These plants are characterized by similar bioactivity. Therefore, this research is aimed at determining the antioxidant and anti-aging properties of A. platyphyllum and A. madurense, as well as identifying the potentially bioactive compounds in these plants. The plant extracts were obtained by successive maceration in n-hexane, ethyl acetate, and methanol in order to increase polarity. The total phenolic, total flavonoid, and total terpenoid contents were quantified using standard methods. Antioxidant activity was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azino-bis-3-ethylbenzothiazoline sulfonate (ABTS) radical scavenging assays. The anti-aging activity was assessed using Schizosaccharomyces pombe yeast cells. The results showed that the methanol extract of A. platyphyllum had the highest phenolic content of 120.06 mg gallic acid equivalent (GAE)/g extract, while the ethyl acetate extract had the highest flavonoid and terpenoid contents of 58.62 mg quercetin equivalent (QE)/g extract and 6.88 mg nerol equivalent (NE)/g extract, respectively. The methanol extracts of the plants showed the highest antioxidant activity of 38.42 mg ascorbic acid equivalent (AAE)/g extract with IC50 value of 91.83 μg/mL. In addition, the methanol extracts showed the highest yeast cell viability, indicating anti-aging potential. The total phenolic content was strongly correlated with the antioxidant activity, suggesting that these substances were essential in the radical scavenging properties of the extracts.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Budiarti E, Batubara I, Ilmiawati A. The potency of Asteraceae plants extracts as antioxidant and antiglycation agent. Jurnal Jamu Indon. 2019; 4(3):103–111.
Oktarina R and Salamah A. Species identification of Asteraceae family at Universitas Indonesia, Depok. J Pro-Life. 2017; 4(1): 241-249.
Jeong KS, Heo TI, Lee KH, Choi K, Kim HJ. A new record Adenostemma madurense DC. (Asteraceae) in Korea. Korean J Plant Res. 2017; 30(3):331-334.
Maeda M, Suzuki M, Fuchino H, Tanaka N, Kobayashi T, Isogai R, Batubara I, Iswantini D, Matsuno M, Kawahara N, Koketsu M, Hamamoto A, Takemori H. Diversity of Adenostemma lavenia, multi-potential herbs, and its kaurenoic acid composition between Japan and Taiwan. J Nat Med. 2022; 76(1):132–143.
Batubara I, Astuti RI, Prastya ME, Ilmiawati A, Maeda M, Suzuki M, Hamamoto A, Takemori H. The anti-aging effect of active fractions and ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic acid isolated from Adenostemma lavenia (L.) O. Kuntze at the cellular level. Antioxid. 2020; 9(8):1–14.
Fauzan A, Praseptiangga D, Hartanto R, Pujiasmanto B. Characterization of the chemical composition of Adenostemma
lavenia (L.) Kuntze and Adenostemma platyphyllum Cass. IOP Conf Ser.: Earth Environ Sci. 2018; 102(1): 012029.
Nur S, Hanafi M, Setiawan H, Nursamsiar N, Elya B. Molecular docking simulation of reported phytochemical compounds from Curculigo latifolia extract on target proteins related to skin anti-aging. Trop J Nat Prod Res. 2023; 7(11):5067-5080.
Sayakti PI, Anisa N, Ramadhan H. Antioxidant activity of methanol extract of cassava leaves (Manihot esculenta Crantz) using CUPRAC method. J Ilm Farm. 2022:97-106.
Andarina R and Djauhari T. Antioksidan dalam dermatologi. J Kedokt dan Kesehat. 2017; 4(1):39-48.
Maesaroh K, Kurnia D, Al Anshori J. Comparison of DPPH, FRAP, and FIC antioxidant activity test methods against ascorbic acid, gallic acid and quercetin. Chim Nat Acta. 2018; 6(2):93-100.
Puspitasari AD, Susanti E, Khustiana A. Antioxidant activity and determination of vitamin C content of lemon juice (Citrus limon (L.) Osbeck) using the ABTS method. J Ilmiah Teknosains. 2019; 5(2):99–104.
Lin SJ and Austriaco N. Aging and cell death in the other yeasts, Schizosaccharomyces pombe and Candida albicans. FEMS Yeast Res. 2014; 14(1):119–135.
Lesmana D, Andrianto D, Astuti RI. Anti-aging properties of the ethanol fractions of clove (Syzygium aromaticum L.) bud and leaf at the cellular levels: Study in yeast Schizosaccharomyces pombe. Sci Pharm. 2021; 89(4):1-14.
Rafi M, Meitary N, Septaningsih DA, Bintang M. Phytochemical profile and antioxidant activity of Guazuma ulmifolia leaves extracts using different solvent extraction. Int J Pharm Pharm Sci. 2020; 31(3):171-180.
Association of Official Analytical Chemists [AOAC]. Official Method of Analysis of The Association of Official Analytical Chemists Nineteenth Ed. Arlington: AOAC International; 2016.
Ministry of Health Republic of Indonesia. Indonesian Herbal Pharmacopoeia. (2nd ed.). Jakarta: Ministry of Health Republic of Indonesia; 2017.
Adelegan AA, Dokunmu TM, Iweala EEJ. In-vitro antioxidant activity and cytotoxic effect of ethanol leaf extract and fractions of Olax subcorpioidea Oliv. (Olacaceae). Trop J Nat Prod Res. 2023; 7(8):3806-3812.
Łukowski A, Jagiełło R, Robakowski P, Adamczyk D, Karolewski P. Adaptation of a simple method to determine the total terpenoid content in needles of coniferous trees. Plant Sci. 2022; 314:111090.
Prastya ME, Astuti RI, Batubara I, Wahyudi AT. Bacillus sp. SAB E-41-derived extract shows anti-aging properties via ctt1-mediated oxidative stress tolerance response in yeast Schizosaccharomyces pombe. Asian Pac J Trop Biomed. 2018; 8(11):533–539.
Ijoma KI, Ajiwe VIE, Odinma ASC. The organic extreact from the leaves of Ficus thonningii Blume, Jatropha tanjorensis J.L Ellis and Saroja and Justicia carnea Lindley as potential nutraceutical antioxidants and functional foods. Trends Phytochem Res. 2023; 7(1):76-85.
Astuti RI, Prastya ME, Batubara I, Budiarti E, Ilmiyawati A. Anti-aging and antioxidant bioactivities of Asteraceae plant fractions on the cellular functions of the yeast Schizosaccharomyces pombe. Adv Pharmacol Pharm Sci. 2021; 2021(2):1-12.
Kurniawati I, Maftuch, Hariati AM. Determination of the best solvent and extract duration on the technique of Gracilaria sp. maceration as well as its influence on moisture content and yield. J Ilmu Perik. 2016; 7(2):72-77.
Yulianti W, Ayuningtyas G, Martini R, Resmeiliana I. Effect of extraction method and solvent polarity on total phenolic content of cherry leaves (Muntingia calabura L). J Sains Terapan. 2020; 10(2):41–49.
Hamka Z, Noena RAN, Azmin RAP. The effect of the multilevel maceration method on the yield value and thin layer chromatography (TLC) profile of basil leaf extract (Ocimum basilicum L.). Jurkes Yamasi Makassar. 2022; 6(1):154-162.
Zhu F. Interactions between starch and phenolic compound. Trends Food Sci Technol. 2015; 43(2):129-143.
Theodora CT, Gunawan IWG, Swantara IMD. Isolation and identification of flavonoid groups in ethyl acetate extract of gedi leaves (Abelmoschus manihot L.). J Chem. 2019; 13(2):131-138.
Verdiana M, Widarta IWR, Permana IDGM. The effect of solvent type in extraction using ultrasonic waves on the antioxidant activity of lemon peel extract (Citrus limon (Linn.) Burm F.). J Ilmu dan Teknologi Pangan. 2018; 7(4):213-222.
Nair NC, Sheela D, Cherian P. HPTLC analysis of flavonoids among selected members of Asteraceae. Int Res J Biol Sci. 2017; 6(6):31-37.
Mutha RE, Tatiya AU, Surana SJ. Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Fut J Pharm Sci. 2021; 7(1):1-13.
Purnama A, Mustanir, Ginting B. Isolation and cytotoxic activity of terpenoid compounds from n-hexane extract of cacao pod husk (Theobroma cacao L.). AIP Conf Proc, Medan; 2021.
Molyneux P. The use of the stable free radical diphenylpicryl hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004; 26(2):211-219.
Chung KW and Chung HY. The effect of calorie restriction on autophagy: Role in aging intervention. Nutr. 2019; 11(12):1-18.
Taormina G and Mirisola MG. Calorie restriction in mammals and simple model organisms. Biomed Res Int. 2014; 308690:1-9.
Fontana L, Partridge L, Longo VD. Dietary restriction, growth factors and aging: from yeast to humans. Sci. 2010; 328(5976):321-326.
Ruetenik A and Barrientos A. Dietary restriction, mitochondrial function and aging: from yeast to humans. BBA – Bioenergetics. 2015; 1847(11): 1434-1447.
Schober P and Schwarte LA. Correlation coefficients: Appropriate use and interpretation. Anesth Analg. 2018; 126(5):1763–1768.