In-vitro and In-silico Studies of a Phenylpropanoid Compound Isolated from Sterculia quadrifida Seeds and Its Inhibitory Effect on Matrix Metalloproteinase-9 http://www.doi.org/10.26538/tjnpr/v7i7.30
Main Article Content
Abstract
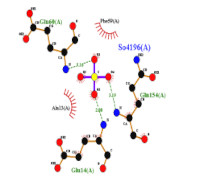
Previous investigation discovered a phenylpropanoid compound called (2E,4E)-1,5-diphenylpenta-2,4-dien-1-one in the chloroform fraction of Sterculia quadrifida seeds. This compound exhibited notable cytotoxic effects, displaying IC50 values of 2.29, 9.93, 18.09, and 12.12 µg/mL in 4T1, MCF-7, MDA-MB-435, and T47D breast cancer cell lines respectively. In our current study, we performed an MMP-9 enzyme inhibition test using the fluorescence resonance energy transfer (FRET) assay and utilized molecular docking and molecular dynamics simulations to explore the compound's mechanism of action. The results revealed that (2E,4E)- 1,5-diphenylpenta-2,4-dien-1-one effectively inhibited the MMP-9 enzyme, achieving a level of 95.78% inhibition with an IC50 value of 34.78 µg/mL. Furthermore, the molecular docking and dynamics simulations indicated robust and stable interactions between (2E,4E)-1,5-diphenylpenta-2,4-dien-1-one and the enzyme's catalytic site. In summary, this study offers significant insights into the development of herbal medicines with promising potential as anticancer drugs.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Hariono M, Nuwarda RF, Yusuf M, Rollando R, Jenie RI, Al-Najjar B, Julianus J, Putra KC, Nugroho ES, Wisnumurti YK, Dewa SP, Jati BW, Tiara R, Ramadani RD, Qodria L,
Wahab HA. Arylamide as Potential Selective Inhibitor for Matrix Metalloproteinase 9 (MMP9): Design, Synthesis, Biological Evaluation, and Molecular Modeling. J Chem Inf
Model. 2020;60(1):349–59.
Hariono M, Rollando R, Karamoy J, Hariyono P, Atmono M, Djohan M, Wiwy W, Nuwarda R, Kurniawan C, Salin N, Wahab H. Bioguided Fractionation of Local Plants against
Matrix Metalloproteinase9 and Its Cytotoxicity against Breast Cancer Cell Models: In Silico and In Vitro Study. Mol Basel Switz. 2020;25(20).
Hariono M, Rollando R, Yoga I, Harjono A, Suryodanindro A, Yanuar M, Gonzaga T, Parabang Z, Hariyono P, Febriansah R, Hermawansyah A, Setyani W, Wahab H.
Bioguided Fractionation of Local Plants against Matrix Metalloproteinase9 and Its Cytotoxicity against Breast Cancer Cell Models: In Silico and In Vitro Study (Part II).
Mol Basel Switz. 2021;26(5):1464.
Hariono M, Yuliani SH, Istyastono EP, Riswanto FDO, Adhipandito CF. Matrix metalloproteinase 9 (MMP9) in wound healing of diabetic foot ulcer: Molecular target and
structure-based drug design. Wound Med. 2018;22:1–13.
Rollando R, Monica E, Afthoni MH, Warsito W, Masruri M, Widodo N. A Phenylpropanoid Compound from the Seeds of Sterculia quadrifida and its Cytotoxic Activity:
http://www.doi.org/10.26538/tjnpr/v7i6.21. Trop J Nat Prod Res TJNPR. 2023;7(6):3203–8.
Rollando R, Warsito W, Masruri M, Widodo N. Potential matrix metalloproteinase-9 inhibitor of aurone compound isolated from Sterculia quadrifida leaves: In-vitro and insilico studies. Res J Pharm Technol. 2022;15(11):5250–4.
Gruszczyk J, Olivares-Illana V, Nourikyan J, Fleurie A, Béchet E, Gueguen-Chaignon V, Freton C, Aumont-Nicaise M, Moréra S, Grangeasse C, Nessler S. Comparative
Analysis of the Tyr-Kinases CapB1 and CapB2 Fused to Their Cognate Modulators CapA1 and CapA2 from Staphylococcus aureus. PLOS ONE. 2013;8(10):e75958.
Li L, Fan P, Chou H, Li J, Wang K, Li H. Herbacetin suppressed MMP9 mediated angiogenesis of malignant melanoma through blocking EGFR-ERK/AKT signaling
pathway. Biochimie. 2019;162:198–207.
Fadana Y, Dinana IA, Srihardyastutie A, Rollando R,Masruri M. Screening Indonesian Pine (Pinus merkusii Jungh at de Vriese) Compound as an Antibacterial Agent: In
Vitro and In Silico Study: http://www.doi.org/10.26538/tjnpr/v7i3.19. Trop J Nat Prod Res TJNPR. 2023;7(3):2586–95.
Rollando R, Maulada F, Afthoni MH, Monica E, Yuniati Y, Nugraha AT. Screening Carica Papaya Compounds as an Antimalarial Agent: In Silico Study:
http://www.doi.org/10.26538/tjnpr/v7i5.9. Trop J Nat Prod Res TJNPR. 2023;7(5):2895–903.
Iskandar D, Widodo N, Warsito W, Masruri M, Rollando R, Warsidah W, Antang YPP. Proposed Functional Activity of Bioactive Compounds from Spatholobus littoralis Hassk in
LC-MS-MS and Silico Studies. Mater Sci Forum. 2022;1061:181–6.
Rollando R, Warsito W, Masruri M, Widodo W. Sterculia foetida Leaf Fraction Against Matrix Metalloproteinase-9 Protein and 4T1 Breast Cancer Cells: In-Vitro and In-Silico
Studies. Trop J Nat Prod Res. 2021;5(1):113–21.
Altharawi A, Riadi Y, Tahir Ul Qamar M. An in silico quest for next-generation antimalarial drugs by targeting Plasmodium falciparum hexose transporter protein: a multipronged approach. J Biomol Struct Dyn. 2023;1–10.
Fonseca AL da, Nunes RR, Braga VML, Comar M, Alves RJ, Varotti F de P, Taranto AG. Docking, QM/MM, and molecular dynamics simulations of the hexose transporterfrom Plasmodium falciparum (PfHT). J Mol Graph Model.
;66:174–86.
Cha H, Kopetzki E, Huber R, Lanzendörfer M, Brandstetter H. Structural Basis of the Adaptive Molecular Recognition by MMP9. J Mol Biol. 2002;320(5):1065–79.
Collier TA, Piggot TJ, Allison JR. Molecular Dynamics Simulation of Proteins. Methods Mol Biol Clifton NJ. 2020;2073:311–27.
Saini G, Dalal V, Savita BK, Sharma N, Kumar P, Sharma AK. Molecular docking and dynamic approach to virtual screen inhibitors against Esbp of Candidatus Liberibacter asiaticus. J Mol Graph Model. 2019;92:329–40.
Tian S, Yu H. Atractylenolide II Inhibits Proliferation, Motility and Induces Apoptosis in Human Gastric Carcinoma Cell Lines HGC-27 and AGS. Mol Basel Switz. 2017;22(11).
Vo TH, Lin YC, Liaw CC, Pan WP, Cheng JJ, Lee CK, Kuo YH. Triterpene glycosides and phenylpropane derivatives from Staurogyne concinnula possessing anti-angiogenic activity. Phytochemistry. 2021;184:112666.
Zhu HL, Qu W, Zhang J, Guo EY, Du T, Liu WY, Cao WY, Feng F, Xu J. Chemical Constituents from Chloranthus anhuiensis and Their Cytotoxic Activities. Chem Biodivers.
;15(10):e1800249.
Hematpoor A, Paydar M, Liew SY, Sivasothy Y, Mohebali N, Looi CY, Wong WF, Azirun MS, Awang K. Phenylpropanoids isolated from Piper sarmentosum Roxb.
induce apoptosis in breast cancer cells through reactive oxygen species and mitochondrial-dependent pathways. Chem Biol Interact. 2018;279:210–8.
Astuti P, Rollando R, Wahyuono S, Nurrochmad A. Antimicrobial activities of isoprene compounds produced by an endophytic fungus isolated from the leaves of Coleus
amboinicus Lour. J Pharm Pharmacogn Res. 2020;8(4):280– 9.