Biological Activities and Crystal Structure Determination of trans, trans-Cyclohexane- 1,2,4,5-tetrol Monohydrate from Pseuduvaria phuyensis (R.M.K. Saunders) http://www.doi.org/10.26538/tjnpr/v7i7.7
Main Article Content
Abstract
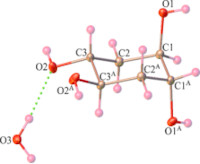
Pseuduvaria phuyensis (Annonaceae), was collected from Kanchanaburi Province, western Thailand. The study aimed to evaluate the antibacterial, anti-HIV-1RT, anti-syncytium (MC99+1A2), and cytotoxic potentials of the crude hexane, ethyl-acetate, and methanol extracts of P. phuyensis leaf-twigs and stems, all in comparison with standard drugs. Also, the isolation and characterization of a compound from one of the active extracts as well as its antibacterial and cytotoxicity activities were carried out. The MIC and MBC values of selected bacterial strains ranged from 3.125 – 200 and 6.25 – 200 mg/mL, respectively for all the extracts, while the isolated compound was not active. The anti-HIV-1 RT and the anti-syncytium assay of the hexane and ethyl acetate extracts revealed they were very active, with % inhibitions of 90.50 and 89.36, respectively. The best inhibitory concentration at 50% (IC50) value was 44.33 µM as exhibited by the hexane extract of the stems of the reverse transcriptase assay, while, the best effective concentration at 50% (EC50) value was 10.4 µM (TI>4.83) as displayed by the ethyl acetate extract of the stems. The cytotoxicity study showed that the hexane extract of the stems displayed high cytotoxicity with ED50 value at 13.07 µg/mL against the FaDu cancer cell line, whil that of the isolated compound was not active. The structure of the isolated compound was characterized and elucidated as trans, trans-cyclohexane-1, 2, 4, 5-tetrol monohydrate using spectroscopic technique and X-ray crystallography analysis.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Johnson DM, Bunchalee P, Chalermglin P, Chantaranothai P, Leeratiwong C, Murray NA, Saunders R, Sirichamorn Y, Su Y, Sutthisaksopon P. Additions to Annonaceae in the Flora of Thailand. Thai Forest Bull., Bot. 2021; 49(2):163- 172. https://li01.tci-thaijo.org/index.php/ThaiForestBulletin/article/view/248520
Couvreur TLP, Pirie MD, Chatro LW, Saunders RMK, Su YCF, Richardson JE, Erkens RHJ. Early evolutionary history of the flowering plant family Annonaceae: steady diversification and boreotropical geodispersal. J. Biogeogr. 2011; 38(4): 664-680.
Al Kazman BSM, Harnett JE, Hanrahan JR. Traditional Uses, Phytochemistry and pharmacological activities of Annonacae. Molecules. 2022; 27(11): 3462. Doi: 10.3390/molecules27113462.
Johnson DM, Bunchalee P, Chalermglin P, Chantaranothai P, Leeratiwong C, Murray NA, Saunders RMK, Sirichamorn Y, Su YCF, Sutthisaksopon P Additions to Annonaceae in the Flora of Thailand. Thai For. Bull. (Bot.). 2021; 49(2): 163-172. https://doi.org/10.20531/tfb.2021.49.2.02
Amudha P, Varadharaj V. Phytochemical and pharmacological potential of Anona species: A review. Asian J. Pharm. Clin. Res. 2017; 10(7): 68-75.
Coria-Téllez AV, Montalvo-Gónzalez E, Yahia EM, ObledoVázquez EN. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. 2018; 11(5):662-691.
https://doi.org/10.1016/j.arabjc.2016.01.004.
Egydio-Brandão APM, Novaes P, Santos DYAC. Alkaloids from Annona: Review from 2005 to 2016. JSM biochem. mol. Boil. 2017; 4(3): 1031(1-12).
Liaw CC, Liou JR, Wu TY, Chang FR, Wu YC. Acetogenins from Annonaceae. Prog Chem Org Nat Prod. 2016; 101: 113- 230. https://doi.org/10.1007/978-3-319-22692-7_2
Novaes P, Pena Ferreira MJ, Santos DYAC. Flavonols from Annona coriacea Mart. (Annonaceae). Biochem. Syst. Ecol. 2018; 78: 77-80. https://doi.org/10.1016/j.bse.2018.04.006
de Moraes MR, Ryan SM, Godoy HT, Thomas AL, Maia J, Richards KM, Tran K, Smith RE. Phenolic compounds and metals in some edible Annonaceae Fruits. Biol. Trace Elem. Res. 2020; 197(2): 676-682. https://doi.org/10.1007/s12011-019-02005-w
Su YCF, Chaowasku T, Saunders RMK. An extended phylogeny of Pseuduvaria (Annonaceae) with descriptions of three new species and a reassessment of the generic status of oreomitra. Syst. Bot. 2010; 35(1): 30-39. http://www.jstor.org/stable/40540523
Saunders RMK, Su YCF, Chalermglin P. Craibella phuyensis (Annonaceae): A New genus and species from Thailand. Syst. Bot. 2004; 29(1):42-49. https://doi.org/10.1600/036364404772974202
Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T, Okitsu T, Fukazawa T, Weber A, Stolz DB, Leboulch P, Tanaka N. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT, Transplantation. 2004; 77(3): 446- 451.
Perera MMN, Dighe SN, Katavic PL, Collet TA. Antibacterial potential of extracts and phytoconstituents isolated from Syncarpia hillii Leaf In Vitro. Plants, 2022; 11(3):283. https://doi.org/10.3390/plants11030283
Pompimon W, Sombutsiri P, Baison W, Udomputtimekakul P, Chuajedton A, Suksen K, Limthongkul J, Naparswad C. Cancer cytotoxic and anti-HIV potential of triphala herb mixture on from Chae Son, Lampang, Thailand. Int. J. Pharm. Res. 2019; 30(6): 1-9.
https://doi.org/10.9734/jpri/2019/v30i630285
Silprasit K, Thammaporn R, Tecchasakul S, Hannongbua S, Choowongkomon K. Simple and rapid determination of the enzyme kinetics of HIV-1 reverse transcriptase and antiHIV-1 agents by a fluorescence based method. J. Virol. Methods. 2011; 171(2): 381-387.
Tan GT, Pezzuto JM, Kinghorn AD, Hughes SH. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J Nat Prod. 1991; 54(1):143-54. https:// doi.org/10.1021/np50073a012.
Kiser R, Makovsky S, Terpening SJ, Laing N, Clanton DJ. Assessment of a cytoprotection assay for the discovery and evaluation of anti-human immunodeficiency virus compounds utilizing a genetically-impaired virus. J Virol Meth. 1996; 58: 99-109.
Nara PL, Hatch WC, Dunlop NM, Robey WG, Arthur LO, Gonda MA, Fischinger PJ. HIV, Perinatal Infections, and Therapy: The Role of the placenta. AIDS Res. Hum. Retrovir. 1987; 3: 283.
Sharma A, Sharma KK. Chemoprotective role of triphala against 1,2-dimethyl hydrazine dihydrochloride induced carcinogenic damage to mouse liver. Indian J. Clin. Biochem. 2011; 26: 290-295.
Sandhya T, Lathika KM, Pandey BN, Mishra KP. Potential of traditional ayurvedic formulation, Triphala, as a novel anticancer drug. Cancer Lett. 2006; 231(2):206-214.
Bruker. APEX3, SAINT-Plus and SADABS. Madison: Bruker AXS Inc.; 2016.
Sheldrick, G.M. (2015) SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallographica Section A, A71, 3-8.
https://doi.org/10.1107/S2053273314026370.
Ramanathan JD, Craigie JS, McLachlan J, Smith DG, McInnes AG. The occurrence of D-(+)-1425- cyclohexanetetrol in monochrysis lutheri droop. Tetrahedron Lett. 1966; 7(14): 1527-1531.
Sheldrick, G.M. (2015) Crystal Structure Refinement with SHELXL. Acta Crystallographica C, C71, 3-8.
https://doi.org/10.1107/S2053229614024218.
Rosatella AA, Afonso CAM. Stereoselective synthesis and isolation of ()-trans, trans-cyclohexane-1, 2, 3, 4-tetratol. AppliedChem. 2022; 2(3): 142-148. https://doi.org/10.3390/appliedchem2030010