A Phenylpropanoid Compound from the Seeds of Sterculia quadrifida and its Cytotoxic Activity http://www.doi.org/10.26538/tjnpr/v7i6.21
Main Article Content
Abstract
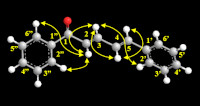
Sterculia quadrifida has been empirically used as a traditional plant for medicinal purposes. The objective of this research is to isolate secondary metabolites present in the seeds of S. quadrifida. Extracts of S. quadrifida seeds were prepared using 80% methanol, and then separated into various fractions using liquid-liquid partition with solvents such as n-hexane, chloroform, ethyl acetate, n-butanol, and an insoluble n-butanol fraction. Preparative HPLC was used to purify the compounds, and their structures were identified through spectroscopic analyses. One phenylpropanoid compound, (2E,4E)-1,5-diphenylpenta-2,4-dien-1-one, was isolated from the chloroform fraction and tested for in vitro cytotoxicity using the MTT assay. The compound demonstrated significant cytotoxic effects, with IC50 values of 2.29, 9.93, 18.09, and 12.12 μg/mL in 4T1, MCF-7, MDA-MB-435, and T47D breast cancer cell lines, respectively. Therefore, the phenylpropanoid compound isolated from S. quadrifida has potential as a cytotoxic agent.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Rollando R, Monica E, Aftoni MH. In vitro Cytotoxic Potential of Sterculia quadrifida Leaf Extract Against Human Breast Cancer Cell Lines:
doi.org/10.26538/tjnpr/v6i8.12. Trop J Nat Prod Res TJNPR. 2022;6(8):1228–32.
Rollando R, Warsito W, Masruri M, Widodo W. Potential Therapeutic Use of Sterculia quadrifida R.Br and Sterculia foetida Linn.: Review. Asian J Plant Sci. 2020;19(4):325–34.
Rollando R, Warsito W, Masruri M, Widodo N. Potential matrix metalloproteinase-9 inhibitor of aurone compound isolated from Sterculia quadrifida leaves: In-vitro and insilico studies. Res J Pharm Technol. 2022;15(11):5250–4.
Rollando R, Warsito W, Masruri M, Nashi W. Antibacterial, Antioxidant, and Cytotoxic Flavonoid Compound from Sterculia Quadrifida Leaves. Trop J Nat Prod Res. 2021;5(11):1979–85.
Rollando R, Engracia M, Monica E, Siswadi S. Immunomodulatory Activity Test of Syrup Dosage Form of Combination Phyllantus niruri Linn. and Sterculia quadrifida R.Br. Extract. Int J Res Pharm Sci. 2020;11(1):191–9.
Akter K, Barnes EC, Brophy JJ, Harrington D, Community Elders Y, Vemulpad SR, Busra RA. Phytochemical Profile and Antibacterial and Antioxidant Activities of Medicinal Plants Used by Aboriginal People of New South Wales, Australia. 2016, Evi-B Comp and Al Med. 2016;11(2):189- 192.
Siswadi S, Saragih GS, Rianawati H, Umroni A, Pujiono E, Setyowati R. Faloak (Sterculia quadrifida R.Br.) bark harvesting for curing hepatitis in Kupang City: Herbal medicine in the urban environment. IOP Conf Ser Earth Environ Sci. 2021;918(1):012014.
Iskandar D, Widodo N, Warsito W, Masruri M, Rollando R, Warsidah W, Antang, YPP. Proposed Functional Activity of Bioactive Compounds from Spatholobus littoralis Hassk in LC-MS-MS and Silico Studies. Mater Sci Forum. 2022;1061:181–6.
Hariono M, Nuwarda RF, Yusuf M, Rollando R, Jenie RI, Al-Najjar B, Julianus J, Putra KC, Nugroho ES, Wisnumurti YK, Dewa SP, Jati BW, Tiara R, Ramadani RD, Qodria L, Wahab HA. Arylamide as Potential Selective Inhibitor for Matrix Metalloproteinase 9 (MMP9): Design, Synthesis, Biological Evaluation, and Molecular Modeling. J Chem Inf Model. 2020;60(1):349–59.
Hariono M, Rollando R, Karamoy J, Hariyono P, Atmono M, Djohan M, Wiwy W, Nuwarda R, Kurniawan C, Salin N, Wahab H. Bioguided Fractionation of Local Plants against Matrix Metalloproteinase9 and Its Cytotoxicity against Breast Cancer Cell Models: In Silico and In Vitro Study. Mol Basel Switz. 2020;25(20).
Hariono M, Rollando R, Yoga I, Harjono A, Suryodanindro A, Yanuar M, Gonzaga T, Parabang Z, Hariyono P, Febriansah R, Hermawansyah A, Setyani W, Wahab H. Bioguided Fractionation of Local Plants against Matrix Metalloproteinase9 and Its Cytotoxicity against Breast Cancer Cell Models: In Silico and In Vitro Study (Part II). Mol Basel Switz. 2021;26(5):1464.
Yao Q, Kong L, Zhang F, Tao X, Li Y. Base-Promoted Tandem Reaction towards Conjugated Dienone or Chromone Derivatives with a Cyano Group: Insertion of Alkynes into C–C σ-Bonds of 3-Oxopropanenitriles. Adv Synth Catal. 2017;359(18):3079–84.
Fadana Y, Dinana IA, Srihardyastutie A, Rollando R, Masruri M. Screening Indonesian Pine (Pinus merkusii Jungh at de Vriese) Compound as an Antibacterial Agent: In Vitro and In Silico Study. Trop J Nat Prod Res TJNPR. 2023;7(3):2586–95.
Mathew B, Oh JM, Abdelgawad MA, Khames A, Ghoneim MM, Kumar S, Sahkar S. Conjugated Dienones from Differently Substituted Cinnamaldehyde as Highly Potent Monoamine Oxidase-B Inhibitors: Synthesis, Biochemistry, and Computational Chemistry. ACS Omega. 2022;7(9):8184–97.
Przydacz A, Kowalczyk R, Albrecht Ł. Brønsted-basecatalyzed remote cascade reactivity of 2,4-dienones – asymmetric synthesis of tetrahydrothiophenes. Org Biomol Chem. 2017;15(45):9566–9.
Nejrotti S, Mannu A, Blangetti M, Baldino S, Fin A, Prandi C. Optimization of Nazarov Cyclization of 2,4-Dimethyl- 1,5-diphenylpenta-1,4-dien-3-one in Deep Eutectic Solvents by a Design of Experiments Approach. Molecules. 2020;25(23):5726.
Zhang S, Neumann H, Beller M. Synthesis of α,β- unsaturated carbonyl compounds by carbonylation reactions. Chem Soc Rev. 2020;49(10):3187–210.
Postolache R, Pérez JM, Castiñeira Reis M, Ge L, Sinnema EG, Harutyunyan SR. Manganese(I)-Catalyzed Asymmetric Hydrophosphination of α,β-Unsaturated Carbonyl Derivatives. Org Lett. 2023;25(10):1611–5.
Budzianowski J, Nawrot J, Nowak G. NMR data for the hydroxyl groups detected by NOESY spectra in sesquiterpene lactones. Data Brief. 2019;25:104246.
Parthasarathy A, Cross PJ, Dobson RCJ, Adams LE, Savka MA, Hudson AO. A Three-Ring Circus: Metabolism of the Three Proteogenic Aromatic Amino Acids and Their Role in the Health of Plants and Animals. Front Mol Biosci. 2018;5(12):29-34.
Rollando R, Yuniati Y, Monica E. Bioactive Potential of Cephalosporium sp. a Fungal Endophyte Isolated from Phyllanthus niruri L. Trop J Nat Prod Res TJNPR. 2023;7(4):2749–55.
Qi SZ, Zhang XX, Jin Y, Wang M, Long LP, Jing WH, Prita RA. Phenylpropanoid-conjugated pentacyclic triterpenoids from the whole plants of Leptopus lolonum induced cell apoptosis via MAPK and Akt pathways in human hepatocellular carcinoma cells. Bioorganic Chem. 2021;111:104886.
Meng DF, Guo LL, Peng LX, Zheng LS, Xie P, Mei Y, Sai HP. Antioxidants suppress radiation-induced apoptosis via inhibiting MAPK pathway in nasopharyngeal carcinoma cells. Biochem Biophys Res Commun. 2020;527(3):770–7.
Rollando R, Maulada F, Afthoni MH, Monica E, Yuniati Y, Nugraha AT. Screening Carica Papaya Compounds as an Antimalarial Agent: In Silico Study. Trop J Nat Prod Res TJNPR. 2023;7(5):2895–903.
Tian S, Wang Z, Wu Z, Wei Y, Yang B, Lou S. Valtrate from Valeriana jatamansi Jones induces apoptosis and inhibits migration of human breast cancer cells in vitro. Nat Prod Res. 2020;34(18):2660–3.
Astuti P, Rollando R, Wahyuono S, Nurrochmad A. Antimicrobial activities of isoprene compounds produced by an endophytic fungus isolated from the leaves of Coleus amboinicus Lour. J Pharm Pharmacogn Res. 2020;8(4):280– 9.
Vo TH, Lin YC, Liaw CC, Pan WP, Cheng JJ, Lee CK, Fung KH. Triterpene glycosides and phenylpropane derivatives from Staurogyne concinnula possessing anti-angiogenic activity. Phytochemistry. 2021 ;184:112666.