Synthesis, Anti-breast Cancer Activity, and Molecular Docking Studies of Thiourea Benzamide Derivatives and Their Complexes with Copper Ion http://www.doi.org/10.26538/tjnpr/v7i6.15
Main Article Content
Abstract
Recently, there has been a rise in interest in the synthesis of chemical compounds with biological activity, especially in the fight against cancer, which is regarded as a modern-day disease. The present study was conducted to synthesize thiourea benzamide derivative ligands and their metal complexes with Cu(II). Thiourea benzamide derivative ligands and their metal complexes with Cu(II) were synthesized. The structures of the synthesized complexes were elucidated by mass spectra, FT-IR, 13C-NMR, and 1H-NMR spectra. Molar conductivity, magnetic susceptibility, SEM, EDX, and TG analyses were also performed. The in vitro anticancer activity of the
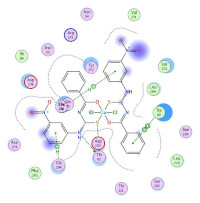
compounds was examined against the breast cancer cell line MCF-7. Molecular docking of complexes (1) and (2) was performed with breast cancer proteins. The synthesized compounds include {[Cu(L)2Cl2].nH2O, where L= ( N-((3-nitrophenyl) carbamothioyl) benzamide (L1, n= 2, (1)), N-(naphthalen-1-ylcarbamothioyl) benzamide (L2, n= 2, (2)), 4-nitro-N-((4-nitrophenyl) carbamothioyl) benzamide (L3, n= 0, (3)), 4-nitro-N-(p-tolylcarbamothioyl) benzamide (L4, n= 2, (4)), N-((4-chlorophenyl) carbamothioyl)-4-nitrobenzamide (L5, n= 4, (5). The ligands behave as bidentate donors and are associated with Cu(II) in a 1:2 (M:L) ratio. Also, the geometric shapes of the prepared complexes were octahedral. The in vitro cellular toxicity evaluation showed that the compounds have low efficacy except for complexes (1) and (2), which have high effectiveness. The molecular docking analysis indicated that the compounds would specifically target PR (PDP: 4OAR) and Akt (PDP: 5KCV) proteins, which had the lowest values of binding energy and
RMSD. The findings of this study reveal that these compounds can be used for therapeutic purposes.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Ili M, Bucos M, Dumitracu F, Cırcu V. Mesomorphic behavior of N-benzoyl-N′-aryl thioureas liquid crystalline compounds. J Mol Str. 2011; 987: 1–6. Doi:10.1016/j.molstruc.2010.11.037
Singh G, Saroa A, Rani S, Girdhar S, Sahoo S, Choquesillo‐Lazarte D. Substituted phenyl urea and thiourea silatranes: Synthesis, characterization, and anion recognition properties by photophysical and theoretical studies. Polyhedron. 2016; 112: 51-60. Doi:10.1016/j.poly.2016.03.036
Saeed A, Ashraf S, White JM, Soria DB, Franca CA, Erben MF. Synthesis, X-ray crystal structure, thermal behavior, and spectroscopic analysis of 1-(1-naphthoyl)-3-(halo-phenyl)-thioureas complemented with quantum chemical calculations, Spectrochimica Acta. 2015; 150: 409-418.
Doi:10.1016/j.saa.2015.05.068
Halim AN, Ngaini Z. Synthesis and characterization of halogenated bis(acylthiourea) derivatives and their antibacterial activities. Phosphorus, Sulfur, and Silicon and the Related Elements. 2017; 192(9): 1012–1017. Doi:10.1080/10426507.2017.1315421
Arslan H, Duran N, Borekci G, Ozer CK, Akbay C. antimicrobial activity of some thiourea derivatives and their nickel and copper complexes. Molec. 2009; 14: 519-527. Doi:10.3390/molecules14010519
Al-Merhj SA. Synthesis and characterization of new thiourea complexes. J Fac Basic Edu. 2012; 73: 45-53.
Ozer CK, Arslan H, VanDerveer D, Binzet G. Synthesis and characterization of N-(alky(aryl) carbamothioyl)cyclohexanecarboxamide derivatives and their Ni(II) and Cu(II) complexes. J Coord Chem. 2009; 62: 266-276. Doi:10.1080/00958970802209623
Stefanska J, Szulczyk D, Koziol AE, Miroslaw B, Kedzierska E, Fidecka S, Busonera B, Sanna G, Giliberti G, La Colla P, Struga M. Disubstituted thiourea derivatives and their activity on CNS: Synthesis and biological evaluation. Eur J Med Chem. 2012; 55: 205-13. Doi:10.1016/j.ejmech.2012.07.020
Shakeel A, Altaf AA, Qureshi AM, Badshah A. Thiourea derivatives in drug design and medicinal chemistry: a short review. J Drug Des Med Chem. 2016; 2(1): 10–20. Doi: 10.11648/j.jddmc.20160201.12
Alkherraz AM, Lusta IZ, Zubi EA. Synthesis and use of thiourea derivative (1-phenyl-3-benzoyl-2-thiourea) for extraction of cadmium ion. Inter Scho Scien Res Innov. 2014; 8(2): 116–118.
Silveira RG, Catão AJ, Cunha BN, Almeida F, Correa RS, Diniz LF, Tenório JC, Ellena J, Kuznetsov AE, Batista AA. Facile synthesis and characterization of symmetric N-[(phenylcarbonyl) carbamothioyl] benzamide thiourea: Experimental and theoretical investigations. J Braz Chem Soc. 2018; 29(12): 2502-2513. Doi:10.21577/0103-5053.20180129
Yamin BM, Yusof MSM, Jusoh RH. 2-Methyl-N-[(3-methyl-2-pyridyl carbamothioylbenzamide. Acta Cryst. E64, 2008; o833. Doi:10.1107/S1600536808009513
Al-Jibori SA, Dayaaf NA, Mohammed MY, Merzweiler K, Wagner Ch, Hogarth G, Richmond MG. cis–trans Isomerism at Square-Planar MN2S2 Centers (M = Pd, Pt): Crystal Structures of N-Phenyl-N-(2-thiazoyl)thiourea Complexes trans-Pd(S2N3C10H8)2 and cis-Pt(S2N3C10H8)2 and Density Functional Calculations. J Chem Crystallogr. 2013; 43: 365–372.Doi:10.1007/s10870-013-0429-7
Pandey SK, Pratap S, Rai SK, Marverti G, Kaur M, Jasinsk JP. Synthesis, characterization, Hirshfeld surface, cytotoxicity, DNA damage and cell cycle arrest studies of N, N-diphenyl-N'-(biphenyl-4-carbonyl/4-chlorobenzoyl) thiocarbamides. J. Mol Struc. 2019; 1186: 333-344. Doi:10.1016/j.molstruc.2019.03.057
Abdel–Hadi KA, Abdel-Razik A, Shoukry MM, Shoheib ShM. Egypt J Chem.1996; 39 (2), 179.
Saeed A, Qamar R, Fattah TA, Flörke U, Erben MF. Recent developments in chemistry, coordination, structure and biological aspects of 1-(acyl/aroyl)-3-(substituted) thioureas. Res Chem Interm. 2017; 43: 3053-3093. Doi:OI 10.1007/s11164-016-2811-5.
Al-Asadi RH, Al-Masoudi WA, Abdu Al-Rassol KS. Synthesis, biological activity, and computational study of some new unsymmetrical organotellurium compounds derived from (2-amino-5-carboxyphenyl) mercury(II) chloride. Asian J Chem. 2016; 28(6): 1171-1176. Doi:10.14233/ajchem.2016.19139.
Rollando R, Monica E, Aftoni MH, In vitro cytotoxic potential of sterculia quadrifida leaf extract against human breast cancer cell lines. Trop J Nat Prod Res. 2022; 6(8):1228-1232 Doi:10.26538/tjnpr/v6i8.12
Scholz C, Knorr S, Hamacher K, Schmidt B. DOCKTITE—A highly versatile step-by-step workflow for covalent docking and virtual screening in the molecular operating environment. J Chem Inf Model. 2015; 55(2): 398–406. Doi:10.1021/ci500681r
Mutlaq DZ, Al-Shawi AA, Al-Asadi RH. Synthesis, characterization, anticancer activity, and molecular docking of novel maleimide–succinimide derivatives. Egypt Pharm J. 2021. Doi: 10.4103/epj.epj_26_21
Elkanzi NA, Ali MA, Albqmi M , Abdou A. New benzimidazole-based Fe (III) and Cr (III) complexes: Characterization, bioactivity screening, and theoretical implementations using DFT and molecular docking analysis. Appl Organomet Chem. 2022; 36(11): e6868. Doi:10.1002/aoc.6868
Faye F, Sylla-Gueye R, Thiam IE, Orton J, Coles S, Gaye M. Synthesis, characterization and crystal structure of 1-(2-benzamidophenyl)-3-benzoylthiourea hemihydrate. Sci J Chem. 2020; 8(6): 131-135. Doi:10.11648/j.sjc.20200806.11
Saad FA. Co-ordination chemistry of some first-row transition metal complexes with multi-dentate ligand (1-benzoyl-3-(4-methylpyridin-2-yl) thiourea), spectral, electrochemical and X – ray single crystal studies. Int J Electrochem Sci. 2014; 9: 4761 – 4775.
Abd Halim AN, Ngaini Z. Synthesis and bacteriostatic activities of bis (thiourea) derivatives with variable chain length. J Chem. 2016; 2739832: 7. Doi:10.1155/2016/2739832
Nawar FA, AL-Asadi RH, Abid DS. Synthesis, antibacterial activity and DFT calculations of some thiazolidine-4-carboxylic acid derivatives and their complexes with Cu(II), Fe(II) and VO(II). Egypt J Chem. 2020. Doi:10.21608/ejchem.2019.16096.1986
Lo SMF, Chui SSY, Shek LY, Lin Z, Zhang XX, Wen GH, Williams ID. Solvothermal synthesis of a stable coordination polymer with copper-i−copper-ii dimer units: [Cu4{1,4-C6H4(COO)2}3(4,4‘-bipy)2]n. J Am Chem Soc. 2000; 122(26): 6293–6294. Doi:10.1021/ja000416c.
Khulbe RC, Singh RP, Bhoon YK. Copper(II), nickel(II) and cobalt(II) complexes of pyridyl and quinolylhydrazones of isatin and methylisatin: Ligand dependent stereochemistry. Trans Met Chem.1983; 8: 59-61. Doi:10.1007/BF00618802
Elkanzi NA , Hrichi H, Salah H, Albqmi M, Ali MA, Abdou A. Synthesis, physicochemical properties, biological, molecular docking and DFT investigation of Fe(III), Co(II), Ni(II), Cu(II) and Zn(II) complexes of the 4-[(5-oxo-4,5-dihydro-1,3-thiazol-2-yl)hydrazono]methyl}phenyl 4-
methylbenzenesulfonate Schiff-base ligand. Inorg Chem Comm. 2023; 148:110331. Doi:10.1016/j.inoche.2022.110331
Bhattacharjee CR, Goswami P, Mondal P. Synthesis, reactivity, thermal, electrochemical and magnetic studies on iron(III) complexes of tetradentate Schiff base ligands. Inorganica Chimica Acta. 2012; 387: 86-92. Doi:10.1016/j.ica.2011.12.056
Gnanamoorthy P, Karthikeyan V, Prabu VA. Field emission scanning electron microscopy (FESEM) characterization of the porous silica nanoparticulate structure of marine diatoms. J Porous Mater. 2014; 21: 225-233. Doi:10.1007/s10934-013-9767-2
Ellingham TD, Thompson TJU, Islam M. Scanning electron microscopy–energy-dispersive X-Ray (SEM/EDX): A rapid diagnostic tool to aid the identification of burnt bone and contested cremains. J Foren Sci. 2017; 63(2): 504-510. Doi:10.1111/1556-4029.13541
Din SU, Iqbal H, Haq S, Ahmad P, Khandaker MU, Elansary HO, Al-Harbi FF, Abdelmohsen ShAM, Zin El-Abedin TK. Investigation of the biological applications of biosynthesized nickel oxide nanoparticles mediated by Buxus wallichiana extract. Crystals. 2022; 12(2): 146.
Doi:10.3390/cryst12020146.
Hoshyar N, Gray S, Han H, Bao Ga. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction, Nanomedicine (Lond). 2016; 11(6): 673–692. Doi: 10.2217/nnm.16.5.
Ji P, Wang P, Chen H, Xu Y, Ge J, Tian Z, Yan Z. Potential of copper and copper compounds for anticancer applications. Pharmaceut. 2023; 16(2): 234. Doi:10.3390/ph16020234
Molinaro C, Martoriati A, Pelinski L, Cailliau K. Copper complexes as anticancer agents targeting topoisomerases i and II. Cancers (Basel). 2020; 12(10): 2863.Doi: 10.3390/cancers12102863
Fagan DH, Fettig LM, Avdulov S, Beckwith H, Peterson MS, Ho YY, Wang F, Polunovsky VA, Yee D. Acquired tamoxifen resistance in MCF-7 breast cancer cells requires hyperactivation of eIF4F-mediated translation. Horm Cancer. 2017; 8(4): 219-229.Doi: 10.1007/s12672-017-0296-3
Venugopal K, Ahmad H, Manikandan E, Thanigai KA, Kavitha K, Moodley M, Rajagopal K, Balabhaskar K, Bhaskar M. The impact of anticancer activity upon Beta vulgaris extract mediated biosynthesized silver nanoparticles (ag-NPs) against human breast (MCF-7), lung (A549) and pharynx (Hep-2) cancer cell lines. J Photochem Photobiol. 2017; 173: 99-107. Doi: 10.1016/j.jphotobiol.2017.05.031.
Dong C, Wu J, Chen Y, Nie J, Chen C. Activation of PI3K/AKT/mTOR pathway causes drug resistance in breast cancer. Frontiers Pharmacol. 2021; 15. Doi Doi:: 10.3389/fphar.2021.628690
Veterini L, Savitri AD, Widyaswari MS, Akbar Muhammad R, Fairus A, Zulfikar MQB, Astri M, Ramasima NA, Anggraeni DP, Nainatika RSA, In silico study of the potential of garlic allicin compound as anti-angiogenesis. Trop J Nat Prod Res. 2021; 5(11):1995-1999
DOi:10.26538/tjnpr/v5i11.17
Alghuwainem YA, Abd El-Lateef HM, Khalaf MM, Abdelhamid AA, Alfarsi A, Gouda M, Abdelbaset M, Abdou A, Synthesis, structural, DFT, antibacterial, antifungal, antiinflammatory, and molecular docking analysis of new VO(II), Fe(III), Mn(II), Zn(II), and Ag(I) complexes based
on 4-((2-hydroxy-1-naphthyl)azo) benzenesulfonamide. J Mol Liq. 2023; 369(1): 120936. Doi:10.1016/j.molliq.2022.120936.