In Silico and In Vitro Study of The Ethanol Extract of The White Garland Lily (Hedychium coronarium J. Koenig) as a Tyrosinase Inhibitor http://www.doi.org/10.26538/tjnpr/v7i6.9
Main Article Content
Abstract
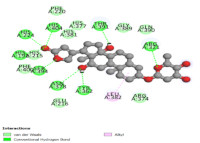
The Zingiberaceae family includes the White Garland Lily (Hedychium coronarium J. Koenig). It is used in traditional medicine for fever, analgesics, and indigestion. In the current study, in silico prediction of the active compound from Hedychium coronarium, analysis of the interaction with a tyrosinase inhibitor, and testing of the tyrosinase inhibitor using dopachrome compounds were done. The software used for docking simulation is Autodock-GPU. Kojic acid solution containing 1000, 500, 250, 125, and 62.5 ppm was measured into a 96-well microtiter dish to which tyrosinase enzyme solution was added, and the pH was adjusted with phosphate buffer. The mixture was incubated at 37ºC for 30 minutes before the absorbance was measured at a wavelength of 492 nm using a microplate reader. Triparanol molecules have the best bonding affinity in the molecular docking simulation data, with a -8.00 kcal/mol value. The extract yield was 3.88%, total ash content of 12.60% ± 0.27%, acid-insoluble ash content of 0.23% ± 0.02%, and water content of 5.96%. Hedychium coronarium has the potential to be developed as a Tyrosinase inhibitor from the result of the insilico investigation. Based on the result, the ethanol extract inhibited the tyrosinase enzyme with an IC50 value of 183.85 ppm compared to kojic acid with an IC50 value of 17.09 ppm.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Solano F. Photoprotection and skin pigmentation: Melaninrelated molecules and some other new agents obtained from natural sources. Molecules. 2020;25(7):1-18
Couteau C, Coiffard L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics. 2016;3(3):1-16
Agorku ES, Kwaansa-Ansah EE, Voegborlo RB, Amegbletor P, Opoku F. Mercury and hydroquinone content of skin toning creams and cosmetic soaps, and the potential risks to the health of Ghanaian women. Springerplus. 2016;5(1):1-5
Talmadge JE, Cowan KH. Gene Therapy in Oncology. Abeloff’s Clin Oncol Fifth Ed. 2014;493-507.e4.
Pillaiyar T, Manickam M, Namasivayam V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J Enzyme Inhib Med Chem. 2017;32(1):403–25.
Li Q, Yang H, Mo J, Chen Y, Wu Y, Kang C, Sun Y, Sun H. Identification by shape-based virtual screening and evaluation of new tyrosinase inhibitors. PeerJ. 2018;2018(1):1-14
Zolghadri S, Bahrami A, Hassan Khan MT, Munoz-Munoz J, Garcia-Molina F, Garcia-Canovas F, Saboury AA. A comprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med Chem. 2019;34(1):279–309.
Panzella L, Napolitano A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: Recent advances. Cosmetics. 2019;6(4):1-13
Pachurekar P, Dixit A. A Review on Pharmacognostical Phytochemical and Ethnomedicinal Properties of Hedychium Coronarium J. Koenig an Endangered Medicine. Int J Chinese Med. 2017;1(2):49–61.
Solano F. On the metal cofactor in the tyrosinase family. Int J Mol Sci. 2018;19(2):1-17
Tavares WR, Barreto M do C, Seca AML. Uncharted Source of Medicinal Products: The Case of the Hedychium Genus. Medicines. 2020;7(5):1-23.
Rachkeeree A, Kantadoung K, Suksathan R, Puangpradab R, Page PA, Sommano SR. Nutritional Compositions and Phytochemical Properties of the Edible Flowers from Selected Zingiberaceae Found in Thailand. Front Nutr. 2018;5(1):1-10
Panigrahy SK, Kumar A, Bhatt R. Hedychium coronarium Rhizomes: Promising Antidiabetic and Natural Inhibitor of α-Amylase and α-Glucosidase. J Diet Suppl. 2020;17(1):1–7.
Hirt PA, Paus R. Healthy Hair (Anatomy, Biology, Morphogenesis, Cycling, and Function). Alopecia. 2019;1–22.
Fu C, Chen J, Lu J, Yi L, Tong X, Kang L, Pei S, Ouyang Y, Jiang L, Ding Y, Zhao X, Li S, Yang Y, Huang J, Zeng Q. Roles of inflammation factors in melanogenesis (Review). Mol Med Rep. 2020;21(3):1421–30.
Kobayashi T, Imokawa G, Bennett DC, Hearing VJ. Tyrosinase stabilization by Tyrp1 (the brown locus protein). J Biol Chem. 1999;273(48):31801–5.
Lai X, Wichers HJ, Soler-Lopez M, Dijkstra BW. Structure of Human Tyrosinase Related Protein 1 Reveals a Binuclear Zinc Active Site Important for Melanogenesis. Angew Chemie - Int Ed. 2017;56(33):9812–5.
Hanwell MD, Curtis DE, Lonie DC, Vandermeerschd T, Zurek E, Hutchison GR. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J Cheminform. 2012;4(8):1-17
Halgren TA. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem. 1996;17(5–6):490–519.
Santos-Martins D, Solis-Vasquez L, Tillack AF, Sanner MF, Koch A, Forli S. Accelerating A uto D ock 4 with GPUs and Gradient-Based Local Search. J Chem Theory Comput. 2021;17(2):1060–73.
Putra PP, Fauzana A, Lucida H. In Silico Analysis of Physical-Chemical Properties, Target Potential, and Toxicology of Pure Compounds from Natural Products. Indones J Pharm Sci Technol. 2020;7(3):107-117.
Asnawi A, Nedja M, Febrina E, Purwaniati P. Prediction of a Stable Complex of Compounds in the Ethanol Extract of Celery Leaves (Apium graveolens L.) Function as a VKORC1 Antagonist. Trop J Nat Prod Res. 2023;7(2):2362–70.
Szklarczyk D, Santos A, Von Mering C, Jensen LJ, Bork P, Kuhn M. STITCH 5: Augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016;44(D1):D380–4.
Howard KM. Platelet-Activating Factor Receptor. Encycl Biol Chem Second Ed. 2013;533–7.
Luu W, Hart-Smith G, Sharpe LJ, Brown AJ. The terminal enzymes of cholesterol synthesis, DHCR24 and DHCR7, interact physically and functionally. J Lipid Res. 2015;56(4):888–97.
Lu P, Jiang Y, Xia Z. Hsa_circ_0003221 facilitates the malignant development of bladder cancer cells via resulting in the upregulation of DHCR24 by targeting miR-892b. Investig Clin Urol. 2022;63(5):577–88.
Long T, Hassan A, Thompson BM, McDonald JG, Wang J, Li X. Structural basis for human sterol isomerase in cholesterol biosynthesis and multidrug recognition. Nat Commun. 2019;10(1):1-8
Song L, Bekdash R, Morikawa K, Quejada JR, Klein AD, Aina-Badejo D, et al. Sigma non-opioid receptor 1 is a potential therapeutic target for long QT syndrome. Nat Cardiovasc Res. 2022;1(2):142–56.
Putra PP, Armin F, Florida N, Yusuf GV, Suharti N. Molecular Dynamics, Prediction of Toxicity, and Interaction of the Active Compound Caesalpinia sappan on Essential Lipids Klebsiella pneumoniae. Adv Heal Sci Res. 2021;302- 309