Molecular Docking Discovered Potential of Cyclooxygenase – 2 Inhibitor Activity of Oily Compounds of Walnuts http://www.doi.org/10.26538/tjnpr/v6i12.8
Main Article Content
Abstract
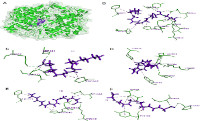
Walnut is a nutritional dietary food containing omega-3 and omega-6 that benefits for the human body. Walnut extract encourages biological properties, including lowering cholesterol, maintaining glucose, and preventing loss of memory and neurodegeneration. This study identified the anti-inflammatory property of walnut fatty acid compounds using molecular docking. Four fatty acid structures of walnut oil, oleic acid, γ – linolenic acid, linolenic acid, and Eicosapentaenoic acid were downloaded from PubChem NCBI database. Those compounds were interacted with cyclooxygenase-2 protein using Molegro Virtual Docker version 5.0, then visualized and analyzed using Discovery studio version 21.1.1. Molecular docking performed several interactions of fatty acids – cyclooxygenase-2 in the active residues. The LYS546, ARG44, THR60, TYR122, GLN543, PRO542, and PHE371 were identified at COX-2 inhibitors (Control) and walnut fatty acids. According to the binding energy, γ-linolenic acid was the lowest binding energy, i.e., -308.0 kJ/mol. Inhibition of fatty acid compounds from walnut extract at inhibitor region reducing cyclooxygenase-2 activities. Low cyclooxygenase-2 expressions might reduce inflammation. This study summarized that the fatty acid compounds of walnut oil promoted anti-inflammatory properties through cyclooxygenase-2 inhibitions.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Danihelová M and Šturdík E. Nutritional and health benefits of buckwheat. Potravinarstvo Slovak J Food Sci. 2012; 6(3):1–9.
Nguyen THD and Vu DC. A Review on Phytochemical Composition and Potential Health-promoting Properties of Walnuts. Food Rev Int. 2021; 1–27.
Ma ZF, Ahmad J, Khan I, Wang CW, Jiang P, Zhang Y. Interaction of Phytochemicals from Walnut on Health: An Updated Comprehensive Review of Reported Bioactivities and Medicinal Properties of Walnut. J Bio Active Prod from Nat.2019; 9(6):410–25.
Singh R and Omre PK. Walnut : Nutritional aspects and by Products – A review. BEPLS. 2018; 7(11):10–2.
Dong FM. The nutritional value of shellfish. J Hygienic Engineering and Design. 2010; (April):10–3.
Jahanban-Esfahlan A, Ostadrahimi A, Tabibiazar M, Amarowicz R. A comparative review on the extraction, antioxidant content and antioxidant potential of different parts of walnut (Juglansregia l.) fruit and tree. Molecules. 2019; 24(11):2133.
Sharma M, Sharma M, Sharma M. A comprehensive review on ethnobotanical, medicinal and nutritional potential of walnut (Juglans regia L.). Proc Indian Natl Sci Acad. 2022; (0123456789).
Copolovici D, Bungau S, Boscencu R, Tit DM, Copolovici L. The fatty acids composition and antioxidant activity of walnut cold press oil. Revista de Chimie. 2017; 68(3):507–9.
Bi D, Zhao Y, Jiang R, Wang Y, Tian Y, and Chen X. Phytochemistry, bioactivity and potential impact on health of Juglans: The original plant of walnut. Nat Prod Communications. 2016; 11(6):869–80.
Kostadinović VS, Naumova LG, Čočevska M, Brühl L, SilaghiDumitrescu R, and Mirhosseini H. Effect of bioactive compounds on antiradical and antimicrobial activity of extracts and coldpressed edible oils from nutty fruits from Macedonia. J Food Meas Charact. 2018; 12(4):2545–52.
Thakur M and Singh K. Walnut ( Juglan regia L .) a completehealth and brain food. Asian J Biol Sci. 2013; 8(2):276-288.
Liu D, Guo Y, Ma H. Production, bioactivities and bioavailability of bioactive peptides derived from walnut origin by-products: a review. Crit Rev Food Sci Nutr. 2022; 1–16.
Sánchez-González C, Ciudad CJ, Noé V, Izquierdo-Pulido M. Health benefits of walnut polyphenols: An exploration beyond their lipid profile. Crit Rev Food Sci Nutr. 2017; 57(16):3373–83.
Delaviz H, Mohammadi J, Ghalamfarsa G, Mohammadi B, Farhadi N. A Review Study on Phytochemistry and Pharmacology Applications of Juglans Regia Plant. Phcog Rev. 2017; 11(22):145–52.
Mirzababaei A, Daneshvar M, Abaj F, Daneshzad E, Hosseininasab D, and Clark CCT. The Effect of Walnut (Juglans regia ) Leaf Extract on Glycemic Control and Lipid Profile in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Clinical Trials . Clin. Nutr. Res. 2022; 11(2):120.
Garcia R, Ferreira JP, Costa G, Santos T, Branco F, Caramona M, et al. Evaluation of anti-inflammatory and analgesic activities of Cymbopogon citratus in vivo-polyphenols contribution. Res J Med Plant. 2015; 9(1):1–13.
Yun BH, Chon SJ, Choi YS, Cho S, Lee BS, Seo SK. Pathophysiology of endometriosis: Role of high mobility group box-1 and toll-like receptor 4 developing inflammation in endometrium. PLoS ONE. 2016; 11(2):1–17.
Suh MG, Choi H-S, Cho K, Park SS, Kim WJ, and Suh HJ.. Anti-inflammatory action of herbal medicine comprised of Scutellaria baicalensis and Chrysanthemum morifolium. Bioscience, biotechnology, and biochemistry. England; 2020; 84(9):1799–809.
Fang J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition. 2015; 31(11–12):1301–6.
Morais CA, Rosso VV de, Estadella D, Pisani LP. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J Nutr Biochem. 2016; 33:1–7.
Kurtys E, Eisel ULM, Hageman RJJ, Verkuyl JM, Broersen LM, Dierckx RAJO, et al. Anti-inflammatory effects of rice bran components. Nut Rev. 2018; 76(5):372–9.
Boukhatem MN, Ferhat MA, Kameli A, Saidi F, Kebir HT. Lemon grass (Cymbopogon citratus) essential oil as a potent antiinflammatory and antifungal drugs. Libyan J Med. 2014; 9.
Patel SS and Savjani JK. Systematic review of plant steroids as potential anti- inflammatory agents: Current status and future perspectives. JPHYTO . 2015; 4(42):121–5.
Amalan V, Vijayakumar N, Indumathi D, Ramakrishnan A. Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed.2016; 84:230–6.
Pradhan B, Nayak R, Patra S, Jit BP, Ragusa A, Jena M. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules (Basel, Switzerland). 2020; 26(1):37.
Oladeji OS, Adelowo FE, Ayodele DT, Odelade KA. Phytochemistry and pharmacological activities of Cymbopogon citratus: A review. Scientific African; 2019; 6:e00137.
Sari DRT, Cairns JRK, Safitri A, Fatchiyah F. Virtual prediction of the delphinidin-3-o-glucoside and peonidin-3-o-glucoside as anti-inflammatory of TNF-α signaling. AIM. 2019; 27(3).
Chandrakirana KG and Fatchiyah F. The Biological Function Prediction of The 10-gingerol Compound of Ginger in Inhibiting Cyclooxygenase-2 Activity. JPACR. 2020; 9(3):222–32.
Prasad S and Tyagi AK. Ginger and Its Constituents: Role in Prevention and Treatment of Gastrointestinal Cancer. Gastroenterol Res Pract. Hindawi Publishing Corporation; 2015; 2015:142979.
Prasad S and Tyagi AK. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol Res Pract. 2015; 2015.
Sari DRT, Krisnamurti GC. 1-dehydrogingerdione, Senyawa Volatil Jahe sebagai Agen Sedatif subtitutif γ - aminobutyrate (GABA); Kajian Biokomputasi. Prosiding Seminar Nasional Biologi. 2021; 7(1):389–95.
Zhang M, Zhao R, Wang D, Wang L, Zhang Q, and Wei S. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytotherapy Research. 2021; 35(2):711–42.
Bare Y, Helvina M, Krisnamurti GC, S M. The Potential Role of 6-gingerol and 6-shogaol as ACE Inhibitors in Silico Study. Biogenesis: Jurnal Ilmiah Biologi. 2020; 8(2):210.
Bare Y, S M, Pili AP, Helvina M. Analysis of molecular interactions of 8-gingerol compounds in Ginger (Zingiber officinale) as ACE Inhibitor. Bioeduscience. 2020; 4(2):183–7.
Bare Y, Sari DRT, Rachmad YT, Krisnamurti GC, Elizabeth A. In Silico Insight the Prediction of Chlorogenic Acid in Coffee through Cyclooxygenase-2 (COX2) Interaction. Biogenesis: Jurnal Ilmiah Biologi. 2019; 7(2):100–5.
Bare Y, Kuki AD, Rophi AH, Krisnamurti GC, Lorenza MRWG, Sari DRT. Prediksi Asam Kuinat Sebagai Anti-Inflamasi Terhadap COX-2 Secara Virtual. Biota : Jurnal Ilmiah Ilmu-Ilmu Hayati. 2019; 4(3):124.
Bare Y, Krisnamurti GC, Elizabeth A, Rachmad YT, Sari DRT, Gabrella Lorenza MRW. The potential role of caffeic acid in coffee as cyclooxygenase-2 (COX-2) inhibitor: In silico study. Biointerface Res. Appl. Chem. 2019; 9(5):4424-4427.
Bare Y, Sari DR, Rachmad YT, Tiring SSND, Rophi AH, Nugraha FAD. Prediction Potential Chlorogenic Acid As Inhibitor Ace (In Silico Study). Bioscience. 2019; 3(2):197.
Bare Y, Timba FS, Putra SHJ, Nirmalasari MY, Sari DRT, Taek MM. Kajian Senyawa Hexose Dan Malic Acid Sebagai Inhibitor Papain Like Protease ( Plpro ) Corona Virus. Biosense. 2022; 05(01):128–37.
Sari DRT and Bare Y. Kajian In Silico Aktivitas Antioksidan Senyawa Bioaktif Dalam Minyak Serai (Cymbopogon Citratus). Al-Kimia. 2021; 9(1):61–9.
Bare Y, Indahsari L, Sari D, Watuguly T. In silico study: Potential prediction of Curcuma longa and Cymbopogon citratus essential oil as Lipoxygenase inhibitor. JSMARTech. 2021; 2(2):075–80.
Mateș L, Popa D-S, Rusu ME, Fizeșan I, Leucuța D. Walnut Intake Interventions Targeting Biomarkers of Metabolic Syndrome and Inflammation in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants (Basel, Switzerland). 2022; 11(7).
Zhi T, Hong D, Zhang Z, Li S, Xia J, and Wang C.. Antiinflammatory and gut microbiota regulatory effects of walnut protein derived peptide LPF in vivo. Food Res. Int. 2022; 152:110875.
Vecchio AJ and Malkowski MG. The structural basis of endocannabinoid oxygenation by cyclooxygenase-2. J Biol Chem;. 2011; 286(23):20736–45.
Bitencourt-Ferreira G and Azevedo WFJ de. Molegro Virtual Docker for Docking. Methods in molecular biology (Clifton, NJ). United States. 2019; 2053:149–67.
Bitencourt-Ferreira G and Azevedo WF de. Docking Screens for Drug Discovery. Methods in Molecular Biology. Ria Grande do Sul: Humana Press; 2019.
Krihariyani D, Wasito EB, Isnaeni I, Siswodihardjo S, Yuniarti WM, Kurniawan E. In Silico Study on Antibacterial Activity and Brazilein ADME of Sappan Wood (Caesalpinia Sappan L.) against Escherichia coli (Strain K12). Sys Rev Pharm. 2020; 11(10):290–6.
Alexanian A and Sorokin A. Cyclooxygenase 2: protein-protein interactions and posttranslational modifications. PHYSIOL GENOMICS. 2017; 49(11):667–81.
Cusimano A, Foderà D, D’Alessandro N, Lampiasi N, Azzolina A, and Montalto G.. Potentiation of the antitumor effects of both selective cyclooxygenase-1 and cyclooxygenase-2 inhibitors in human hepatic cancer cells by inhibition of the MEK/ERK pathway. Cancer Biol Ther. 2007; 6(9):1461–8.
Desai SJ, Prickril B, Rasooly A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr Cancer. 2018; 70(3):350–75.
Wilgus TA, Bergdall VK, Tober KL, Hill KJ, Mitra S, and Flavahan NA. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am J Pathol. 2004; 165(3):753–61.
Frejborg E, Salo T, Salem A. Role of Cyclooxygenase-2 in Head and Neck Tumorigenesis. Int J Mol Sci. 2020; 21(23).
Nanda N and Dhawan DK. Role of Cyclooxygenase-2 in colorectal cancer patients. Frontiers in bioscience (Landmark edition). Singapore; 2021; 26(4):706–16.
Chebrolu A and Madhavan S. Molecular Docking Study of Ibuprofen Derivatives as Selective Inhibitors of Cyclooxygenase-2. IJPSR. 2020; 11(12):6526–31.
Bhagavat R, Saqib A, Karigar C. Molecular docking studies of novel palmitoyl-ligands for cyclooxygenase-2. Chem Biol Drug Des. England. 2012.
Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, et al. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm Res. 2009; 26(11):2438–45.
Hussain S, Guruvayoorappan C, Komal KP, Ennaganti S. Molecular docking analysis of doronine derivatives with human COX-2. Bioinformation. 2020; 16(6):483–92.
Khan MF, Nabila S, Rashid R, Rahman M, Chowdhury A, Rashid M. In Silico Molecular Docking Studies of Lichen Metabolites Against Cyclooxygenase-2 Enzyme. Bangladesh JPharmacol. 2015; 18:90–6.
Amaravani M, Prasad NK, Ramakrishna V. COX-2 structural analysis and docking studies with gallic acid structural analogues. SpringerPlus. 2012; 1(1):58.
Tendulkar R and Mahajan S. Molecular Docking studies of Novel Flavones as Cyclooxygenase-2 (Cox 2) Inhibitors. JAPER. 2014; 4(3):330–8.
Moussa N, Hassan A, Gharaghani S. Pharmacophore model, docking, QSAR, and molecular dynamics simulation studies of substituted cyclic imides and herbal medicines as COX-2 inhibitors. Heliyon. 2021; 7(4):e06605.
Bare Yohanes, Sari DRT, Ujiana Wa Ode, Ra’O, PYS, Pada K. Kajian In Silico 6-Paradol Sebagai Herbal Alternatif Pengobatan Penyakit Alzheimer. Med Sains. 2022; 7(2):1–8.
Sari DRT, Krisnamurti GC, Bare Y. Pemetaan Bioaktivitas Senyawa Metabolit Sekunder Pada Kayu Secang (Caesalpinia sappan) Secara In Silico. J Pharmasci (J. of Pharm. and Sci.). 2022; 7(1):21–8.
Sari DRT and Bare Y. Physicochemical properties and biological activity of bioactive compound in Pepper nigrum: In silico study. Spizaetus: J Bio dan Pendidikan Biologi. 2020; 1(1):1–6.
Rophi AH, Bare Y, Sari DRT. The Potential of Acetylfuran and Furfural from Tamarindus indica as Lipoxygenase Inhibitor: In Silico Study Apriani. J Farmasi Dan Ilmu Kefarmasian Indonesia. 2021; 8(2):162–7.