Thin Layer Chromatography - Direct Bioautography and Identification of Compounds from the Semi-purified Fraction of Senna alata (Linn.)
Main Article Content
Abstract
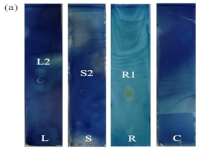
The availability and affordability of medicinal plants, along with the fact that certain bacteria are resistant to synthetic drugs, have led to increased interest in natural products derived from plants for medical applications. Senna alata (Linn.), commonly known as “gelenggang” and “daun kurap”, has demonstrated a wide range of antibacterial properties against infections caused by bacterial pathogens. This study aimed to isolate and identify the bioactive compounds present in the leaf, stem, and root of Cassia alata, and to evaluate their antimicrobial activities. Thin Layer Chromatography-Direct Bioautography (TLC-DB) and Gas Chromatography-Mass Spectrometry (GC-MS) techniques were utilized for compound separation and identification. In the TLC separation, four, seven, and five spots were observed on the TLC plates for the leaf, stem, and root extracts, respectively. The bacterium Mammaliicoccus sp. exhibited a strong inhibition zone corresponding to one distinct spot on each TLC-DB plate: leaf (Rf = 0.47), stem (Rf = 0.40), and root (Rf = 0.42). In contrast, Enterococcus sp. showed a weaker inhibition at those same spots. GC-MS analysis of the active spots identified major bioactive compounds, including Phenol, 3,5-bis(1,1-dimethylethyl)-, Cholest-5-en-3-ol (3.beta.)-, carbonochloridate, Neophytadiene, Dodecane, 2,6,11-trimethyl-, 2-Pentadecanone, 6,10,14-trimethyl-, and Decane, 3,7-dimethyl-,. These findings suggest that Senna alata contains bioactive compounds with notable antimicrobial properties, supporting its potential application in developing alternative treatments for infections caused by Mammaliicoccus sp. and Enterococcus sp. This study highlights the importance of further investigating plant-derived compounds as promising candidates in the search for new antimicrobial agents.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
⦁ Idemudia OU, Enogieru AB. Phytochemical and pharmacological activities of Cucumis sativus: An updated review. Trop J. Nat Prod Res. 2024; 8(7):7612-7623. DOI: https://doi.org/10.26538/tjnpr/v8i7.1
⦁ Md Farid FF, Bakar FIA, Abdullah N, Mohamad A, Hanafi AFM, Wahyuni AS. Medicinal benefits of allicin in black garlic and its potential impact on post-harvest degradation: A review. Trop J. Nat Prod Res. 2024; 8(7):7624-7638. DOI: https://doi.org/10.26538/tjnpr/v8i7.2
⦁ Vaou N, Stavropoulou E, Voidarou C, Tsigalou C, Bezirtzoglou E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms. 2021; 9(10):1-28. DOI: https://doi.org/10.3390/microorganisms9102041
⦁ Madubuobi OG, Lawal-Sanni AO, Ajoseh SO, Salami WO, Ukhureigbe IM, Akinyemi KO. Detection and screening of some medicinal plants against multiple drug-resistant Pseudomonas aeruginosa from selected sources. Trop J. Nat Prod Res. 2024; 8(7):7845-7854. DOI: https://doi.org/10.26538/tjnpr/v8i7.31
⦁ Pöntinen AK, Top J, Arredondo-Alonso S, Tonkin-Hill G, Freitas AR, Novais C, Corander J. Apparent nosocomial adaptation of Enterococcus faecalis predates the modern hospital era. Nat Commun. 2021; 12(1):1223-1536. DOI: https://doi.org/10.1038/s41467-021-21749-5
⦁ Fodor A, Abate BA, Deák P, Fodor L, Gyenge E, Klein MG, Makrai L. Multidrug resistance (MDR) and collateral sensitivity in bacteria, with special attention to genetic and evolutionary aspects and to the perspectives of antimicrobial peptides - A review. Pathogens. 2020; 9(7):1-55. DOI: https://doi.org/10.3390/pathogens9070522
⦁ Ruiz-Romero RA, Vargas-Bello-Pérez E. Non-aureus staphylococci and mammaliicocci as a cause of mastitis in domestic ruminants: Current knowledge, advances, biomedical applications, and future perspectives - a systematic review. Vet Res Commun. 2023; 47(3):1067-1084. DOI: https://doi.org/10.1007/s11259-023-10090-5
⦁ Dhaouadi S, Soufi L, Campanile F, Dhaouadi F, Sociale M, Lazzaro L, Cherif A, Stefani S, Elandoulsi RB. Prevalence of methicillin-resistant and -susceptible coagulase-negative staphylococci with the first detection of the mecC gene among cows, humans and manure in Tunisia. Int J. Antimicrob Agents. 2020; 55(1):105826-105831. DOI: https://doi.org/10.1016/j.ijantimicag.2019.10.007
⦁ Oladeji OS, Adelowo FE, Oluyori AP, Bankole DT. Ethnobotanical description and biological activities of Senna alata. Evid Based Complement Altern Med. 2020; (1): 2580259-2580271. DOI: https://doi.org/10.1155/2020/2580259
⦁ Ogba OM, Udoh DI, Udonkang MI, Eyo A-AO, Chukwueke SE, Eshemitan Z, Akpan NG. Antimicrobial effect of Cassia alata leaf extracts on fungal isolates from Tinea infections. Trop J. Nat Prod Res. 2023; 7(5):3034-3037. DOI: https://doi.org/10.26538/tjnpr/v7i5.29
⦁ Bakar FA, Razzaq KW, Ahmad KI, Magiman MM, Rosli Z, Seemab A, Faridah-Hanum I. Diversity and utilization of ethnomedicinal plants in Sarawak, Borneo. Malays For 2023; 86(1):125–152.
⦁ Fazwa F, Norhayati S, Syafiqah Nabilah SB, Keeren SR. Selection of high yielding genotypes of Senna alata for future. Int J. Agric For Plant. 2020; 10:379–385.
⦁ Saidin SH, Azah N, Ali M, Hirmizi NM, Yusoff N, Abdullah Z, Markandan S, Khoo M, Pisar M, Jamil M, Lee TA, Hashim N, Mohamed S, Caadir S. Skin care active ingredients from Senna alata (L.) Roxb extracts. Asian J. Pharmacog. 2019; 3(1):21-29.
⦁ Tatsimo SJN, Tsague VT, Lamshoft M, Sarkar P, Bag PK, Spiteller M. Antibacterial-guided isolation of constituents from Senna alata leaves with a particular reference against multi-drug-resistant Vibrio cholerae and Shigella flexneri. Int J. Biol Chem Sci. 2017; 11(1):46-53. DOI: https://doi.org/10.4314/ijbcs.v11i1.4
⦁ Ita BN, Ndukwe GI. Antioxidant activity of Senna alata root extracts. J. Nat Prod Resour. 2017; 3(1):94–96.
⦁ Toh SC, Lihan S, Bunya SR, Leong SS. In vitro antimicrobial efficacy of Cassia alata (Linn.) leaves, stem, and root extracts against cellulitis causative agent Staphylococcus aureus. BMC Complement Med Ther. 2023; 23(85): 1-17. DOI: https://doi.org/10.1186/s12906-023-03914-z
⦁ Chukwuemerie OL, Bunu SJ, Iloh ES, Ogujiuba CU, Obasi J, Onwuzuluigbo CC, Okeke CO. Evaluation of the anthelmintic property of the endophytic extract of Senna alata in mice model. J. Med Plants Stud. 2022; 10(3): 52–54. DOI: https://doi.org/10.22271/plants.2022.v10.i3a.1425
⦁ Albert V, Ransangan J. Antibacterial potential of plant crude extracts against Gram negative fish bacterial pathogens. Int J. Pharm Res Bio-Sci. 2013; 3(2):21–27.
⦁ Mulat M, Pandita A, Khan F. Medicinal plant compounds for combating the multi-drug resistant pathogenic bacteria: A review. Curr Pharm Biotechnol. 2019; 20(3):183–196. DOI: https://doi.org/10.2174/1872210513666190308133429
⦁ Valle DL, Puzon JJM, Cabrera EC, Rivera W. Thin layer chromatography-bioautography and gas chromatography-mass spectrometry of antimicrobial leaf extracts from Philippine Piper betle L. against multidrug-resistant bacteria. Evid Based Complement Altern Med. 2016; 1–7. DOI: https://doi.org/10.1155/2016/4976791
⦁ Parys W, Dołowy M, Pyka-Pająk A. Significance of chromatographic techniques in pharmaceutical analysis. Processes. 2022; 10(1):172-210. DOI: https://doi.org/10.3390/pr10010172
⦁ Odeyemi S, Afolayan A, Bradley G. Phytochemical analysis and anti-oxidant activities of Albuca bracteata Jacq. and Albuca setosa Jacq bulb extracts used for the management of diabetes in the Eastern Cape, South Africa. Asian Pac J. of Trop Biomed. 2017; 7(6):577–584. DOI: https://doi.org/10.1016/j.apjtb.2017.05.013
⦁ Zaini AS, Aris NA, Putra NR, Abd Hashib S, Kamaruddin MJ, Idham Z, Che Yunus MA. Comparison of charantin extract from Momordica charantia using modified supercritical carbon dioxide and soxhlet extraction method. Mal J. Fund Appl Sci. 2018; 14(4):462–466. DOI: https://doi.org/10.11113/mjfas.v14n4.1092
⦁ Ogunlaja OO, Moodley R, Baijnath H, Jonnalagadda SB. Antioxidant activity of the bioactive compounds from the edible fruits and leaves of Ficus sur Forssk. (Moraceae). S Afr J. Sci. 2022; 118(3–4):1-5. DOI: https://doi.org/10.17159/sajs.2022/9514
⦁ Cahyaningrum SE, Muhaimin FI, Lestari NRD, Amaria. A novel gel combination of Binahong leaf extract, aloe vera, chitosan, and nanosilver as antibacterial agent against Staphylococcus aureus. Trop J. Nat Prod Res. 2025; 9(6):2395-2401. DOI: https://doi.org/10.26538/tjnpr/v9i6.6
⦁ Jesionek W, Móricz ÁM, Ott PG, Kocsis B, Horváth G, Choma IM. TLC-direct bioautography and LC/MS as complementary methods in identification of antibacterial agents in plant Tinctures from the Asteraceae family. J. AOAC Int. 2015: 98(4):857-861. DOI: https://doi.org/10.5740/jaoacint.SGE2-Choma
⦁ Dar AA, Verma NK, Arumugam N. An updated method for isolation, purification and characterization of clinically important antioxidant lignans - sesamin and sesamolin, from sesame oil. Ind Crops Prod. 2015; 64:201–208. DOI: https://doi.org/10.1016/j.indcrop.2014.10.026
⦁ Samling BA, Assim Z, Tong WY, Leong CR, Ab Rashid S, Nik Mohamed Kamal NNS, Muhamad M, Tan WN. Cynometra cauliflora L.: An indigenous tropical fruit tree in Malaysia bearing essential oils and their biological activities. Arab J. Chem. 2021; 14(9):103302-103314. DOI: https://doi.org/10.1016/j.arabjc.2021.103302
⦁ Kowalska T, Sajewicz M. Thin-layer chromatography (TLC) in the screening of botanicals-its versatile potential and selected applications. Molecules. 2022; 27(19):6607-6630. DOI: https://doi.org/10.3390/molecules27196607
⦁ Sanjeet K, Jyotirmayee K, Monalisa S. Thin layer chromatography: A tool of biotechnology for isolation of bioactive compounds from medicinal plants. Int J. Sci Rev. 2013; 18(1):126–132.
⦁ Belaqziz M, Tan SP, El-Abbassi A, Kiai H, Hafidi A, O’Donovan O, McLoughlin P. Assessment of the antioxidant and antibacterial activities of different olive processing wastewaters. PLoS ONE. 2017; 12(9):1-16. DOI: https://doi.org/10.1371/journal.pone.0182622
⦁ Mundy L, Pendry B, Rahman M. Antimicrobial resistance and synergy in herbal medicine. J. Herb Med. 2016; 6(2):53–58. DOI: https://doi.org/10.1016/j.hermed.2016.03.001
⦁ Ndiege ML, Kengara F, Maiyoh GK. Characterization of phenolic compounds from leaf extracts of Bidens pilosa L. Var. Radiata. South Asian Res J. Nat Prod. 2021; 4(3):44–58.
⦁ Novian DR. Anthelmintic potential of Moringa oleifera as inhibitor mitochondrial rhodoquinol-fumarate reductase from Ascaris suum using the docking method. J. Pharm Sci Prac. 2019; 5(2):106-114.
⦁ Igwe KK, Okafor PN, Ijeh II. GC-MS analysis of phytocomponents in Vernonia amygdalina. Del leaves and its contractile potential in mammary tissue in female albino Wistar rats. IOSR J. Agricul Vet Sci. 2015; 8(11):2319–2372.
⦁ Maulidia V, Soesanto L, Syamsuddin Khairan K, Hamaguchi T, Hasegawa K, Sriwati R. Secondary metabolites produced by endophytic bacteria against the root-knot nematode (Meloidogyne sp.). Biodiversitas 2020; 21(11):5270–5275. DOI: https://doi.org/10.13057/biodiv/d211130
⦁ Santhi V, Sivakumar V, Jayalakshmi S, Thilaga RD, Mukilarasi M. Isolating bioactive compound from marine Prosobranch Purpura persica from Tuticorin Coast. Int J. Env Prot Policy. 2016; 4(3):64-76. DOI: https://doi.org/10.11648/j.ijepp.20160403.14
⦁ Bakrim S, Benkhaira N, Bourais I, Benali T, Lee LH, El Omari N, Sheikh RA, Goh KW, Ming LC, Bouyahya A. Health benefits and pharmacological properties of stigmasterol. Antioxidants. 2022; 11(10):1912-1944. DOI: https://doi.org/10.3390/antiox11101912
⦁ Khanal LN, Sharma KR, Pokharel YR, Kalauni SK. Characterization of essential oil, estimation of phenolic and flavonoid content and biological activities of Ephedra pachyclada BOISS. J. Inst Sci Tech. 2022; 27(1):27-35. DOI: https://doi.org/10.3126/jist.v27i1.40590
⦁ Tiji S, Rokni Y, Benayad O, Laaraj N, Asehraou A, Mimouni M. Chemical composition related to antimicrobial activity of Moroccan nigella sativa L. extracts and isolated fractions. Evid Based Complement Altern Med. 2021; (1): 8308050-8308064. DOI: https://doi.org/10.1155/2021/8308050
⦁ Fatmawati S, Purnomo AS, Bakar MFA. Chemical constituents, usage and pharmacological activity of Cassia alata. Heliyon. 2020; 6(7):1-11. DOI: https://doi.org/10.1016/j.heliyon.2020.e04396
⦁ Jaradat N, Ghanim M, Abualhasan MN, Rajab A, Kojok B, Abed R, Mousa A, Arar M. Chemical compositions, antibacterial, antifungal and cytotoxic effects of Alhagi mannifera five extracts. J. Complement Integr Med. 2022; 19(4):869-877. DOI: https://doi.org/10.1515/jcim-2021-0206
⦁ Singh N, Mansoori A, Jiwani G, Solanke AU, Thakur TK, Kumar R, Chaurasiya M, Kumar A. Antioxidant and antimicrobial study of Schefflera vinosa leaves crude extracts against rice pathogens. Arab J. Chem. 2021; 14(7):103243-103255. DOI: https://doi.org/10.1016/j.arabjc.2021.103243
⦁ Ariole CN, Agulanna EU. Bioprospecting of an indigeneous Bacillus thuringiensis strain G5-8-3T02 for shrimp culture system. South Asian J. Res Microbiol. 2020; 6(1):33-43. DOI: https://doi.org/10.9734/sajrm/2020/v6i130142
⦁ Sargunam JH, Thilakavathy S. GCMS profile of bioactive compounds with therapeutic potential in Beta vulgaris (L.) ethanolic leaf extracts. J. Pharm Res Int. 2021; 33:354-360. DOI: https://doi.org/10.9734/jpri/2021/v33i43B32563
⦁ Ahsan T, Chen J, Zhao X, Irfan M, Wu Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express. 2017; 7(54):1-9. DOI: https://doi.org/10.1186/s13568-017-0351-z
⦁ Al-Rajhi, AM, Qanash H, Almuhayawi MS, Al Jaouni SK, Bakri MM, Ganash M, Salama HM, Selim S, Abdelghany TM. Molecular interaction studies and phytochemical characterization of Mentha pulegium L. constituents with multiple biological utilities as antioxidant, antimicrobial, anticancer and anti-hemolytic agents. Molecules. 2022; 27(15):4824-4850. DOI: https://doi.org/10.3390/molecules27154824
⦁ Amos-tautua BM, Alayande KA, Ajileye O, Fadare OA, Onigbinde A, Songca SP. Effect of the leaf extracts of Funtumia africana (Benth.) Stapf. against selected pathogens. J. Med Plants Stud. 2020; 8(4):125-129.
⦁ Zayed MZ, Samling B. Phytochemical constituents of the leaves of Leucaena leucocephala from Malaysia. Int J. Pharm Pharm Sci. 2016; 8(12):174-179. DOI: https://doi.org/10.22159/ijpps.2016v8i12.11582
⦁ Kim DH, Park MH, Choi YJ, Chung KW, Park CH, Jang EJ, An HJ, Yu BP, Chung HY. Molecular study of dietary heptadecane for the anti-inflammatory modulation of NF-kB in the aged kidney. PLOS ONE. 2013; 8(3):1-10. DOI: https://doi.org/10.1371/journal.pone.0059316
⦁ Addai, ZR, Abood MS, Hlail SH. GC-MS profiling, antioxidants and antimicrobial activity of prickly pear (Opuntiaficus-indica) pulp extract. Pharmacogn J. 2022; 14(2):262-267. DOI: https://doi.org/10.5530/pj.2022.14.32
⦁ Gomathi S, Velayutham P, Karthi C, Santhoshkumar S. In vitro callus induction and phytochemical screening of
⦁ Corbichonia decumbens (Forssk.) Exell through GC-MS analysis. J. Pharmacog Phytochem. 2019; 8(5):566-571.
⦁ Arora S, Kumar G. Phytochemical screening of root, stem and leaves of Cenchrus biflorus Roxb. J. Pharmacog Phytochem. 2018; 7(1):1445-1450.
⦁ Zhu YZ, Liu JW, Wang X, Jeong IH, Ahn YJ, Zhang CJ. Anti-BACE1 and antimicrobial activities of steroidal compounds isolated from marine Urechis unicinctus. Mar Drugs. 2018; 16(3):94-106. DOI: https://doi.org/10.3390/md16030094
⦁ Tan WN, Shahbudin FN, Mohamed Kamal NNSN, Tong WY, Leong CR, Lim JW. Volatile constituents of the leaf essential oil of Crinum asiaticum and their antimicrobial and cytotoxic activities. J. Essent Oil-Bear Plants. 2019; 22(4):947–954. DOI: https://doi.org/10.1080/0972060X.2019.1683079