Enhancing Solubility and Dissolution Rate of p-Methoxycinnamic Acid via Multicomponent Crystal Formation with Meglumine
Main Article Content
Abstract
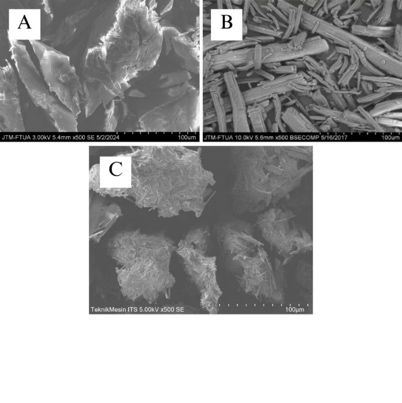
p-Methoxycinnamic acid, a bioactive compound derived from the hydrolysis of ethyl p-methoxycinnamate found in the rhizome of Kaempferia galanga, possesses notable pharmacological activity, including analgesic, anti-inflammatory, and antidiabetic properties. However, its poor water solubility limits its effectiveness, necessitating high doses to achieve therapeutic levels. As a Biopharmaceutics Classification System (BCS) class II compound, it has low solubility but high permeability, making solubility and dissolution rate the main barriers to absorption. This study addresses these limitations by forming a multicomponent crystal with meglumine as a coformer to enhance its solubility and therapeutic potential. The multicomponent crystal was prepared in a 1:1 molar ratio using the solvent drop grinding method. Solid state characterization was performed using differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), Fourier transform infrared spectroscopy (FT-IR), and scanning electron microscopy (SEM). PXRD analysis revealed new diffraction peaks, indicating the formation of a distinct crystalline phase, while FT-IR spectra confirmed molecular interactions through wavenumber shifts. SEM analysis illustrated significant morphological changes in the multicomponent crystal compared to the pure constituent. Solubility of the multicomponent crystal showed a 3.4-fold increase, and dissolution studies demonstrated a 2.6-fold enhancement in dissolution efficiency compared to pure p-methoxycinnamic acid. These results highlight the successful formation of a p-methoxycinnamic acid-meglumine multicomponent crystal, classified as a salt. This approach not only improves solubility and dissolution efficiency but also provides a practical strategy for enhancing the formulation of poorly water-soluble drugs. These improvements may lead to better clinical performance and therapeutic outcomes, making it a valuable option in pharmaceutical development.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. HMDB. The Metabolomics Innovation Centre. Metabocard for 4-methoxycinnamic acid. [Internet]. TMIC. 2023. Available from: https://hmdb.ca/metabolites/HMDB0002040
2. Isadiartuti D, Ekowati J, Noorma, Rosita, Amalia NR. The dissolution of p-methoxycinnamic acid-β-cyclodextrin inclusion complex produced with microwave irradiation. Vol. 14, JPHIA. 2023. p. 1–7. https://doi.org/10.4081/jphia.2023.2500 DOI: https://doi.org/10.4081/jphia.2023.2500
3. Sulistyowaty MI, Setyawan D, Sari R, Paramanandana A, Maharani NA, Simorangkir TP. Preparation and Physicochemical Characterizations of p-Methoxycinnamic acid – Succinic Acid Cocrystal by Solvent Evaporation Technique. J Med Sci. 2022;10(A):1444–1449. http://dx.doi.org/10.3889/oamjms.2022.10193 DOI: https://doi.org/10.3889/oamjms.2022.10193
4. Sulistyowaty MI, Sari IP, Yusuf H, Zaini E. The formation of p-Methoxycinnamic acid-caffeine co-crystal by the solution evaporation method and its physicochemical characterization. AIP Conf Proc. 2023;1(1):1–9. https://doi.org/10.1063/5.0119975 DOI: https://doi.org/10.1063/5.0119975
5. Fitriani L, Fadina H, Usman H, Zaini E. Formation and Characterization of Multicomponent Crystal of Trimethoprim and Mandelic Acid By Solvent Drop Grinding Method. Int J Appl Pharm. 2023;15:75–79. http://dx.doi.org/10.22159/ijap.2023.v15s1.06 DOI: https://doi.org/10.22159/ijap.2023.v15s1.06
6. Singh M, Barua H, Jyothi VGSS, Dhondale MR, Nambiar AG, Agrawal AK, Kumar P, Shastri NR, Kumar D. Cocrystals by Design : A Rational Coformer Selection Approach for Tackling the API Problems. Pharmaceutics. 2023;15(1161):1–43. https://doi.org/10.3390/pharmaceutics15041161 DOI: https://doi.org/10.3390/pharmaceutics15041161
7. Rong Y, Xue S, Li S, Pang S. Study on Preparation of Pillararene Cocrystals by Liquid-Assisted Grinding. J Phys Conf Ser. 2023;2539(1):1. http://dx.doi.org/10.1088/1742-6596/2539/1/012050 DOI: https://doi.org/10.1088/1742-6596/2539/1/012050
8. Rodrigues ACC, Sallum LO, Aguiar ASN, Camargo AJ, Napolitano HB. Exploring the Aqueous Solubility and Intermolecular Interactions of Diclofenac Diethylammonium: A Molecular Modeling Study in Solid State and Solvation Processes. J Mol Liq. 2024;401(124613):0167–7322. https://doi.org/10.1016/j.molliq.2024.124613 DOI: https://doi.org/10.1016/j.molliq.2024.124613
9. Jessica A, Yasa SWN, Zaini E, Fitriani L. Increased Dissolution Rate of Aceclofenac by Formation of Multicomponent Crystals With L-Glutamine. Int J Appl Pharm. 2024;16:45–52. http://dx.doi.org/10.22159/ijap.2024.v16s1.09 DOI: https://doi.org/10.22159/ijap.2024.v16s1.09
10. Xia N, Liu Y, Gao D, Zhu S. Molecular Interaction and Solubilization Efficiency of Neohesperidin in Ternary Systems with Hydroxypropyl-β-cyclodextrin and Meglumine. Foods. 2024. 13; (19):3143. https://doi.org/10.3390/foods13193143 DOI: https://doi.org/10.3390/foods13193143
11. Aloisio C, Antimisiaris SG, Longhi MR. Liposomes containing cyclodextrins or meglumine to solubilize and improve the bioavailability of poorly soluble drugs. J Mol Liq. 2017;229:106–13. Available from: http://dx.doi.org/10.1016/j.molliq.2016.12.035 DOI: https://doi.org/10.1016/j.molliq.2016.12.035
12. Aloisio C, de Oliveira AG, Longhi M. Solubility and release modulation effect of sulfamerazine ternary complexes with cyclodextrins and meglumine. J Pharm Biomed Anal. 2014;100:64–73. http://dx.doi.org/10.1016/j.jpba.2014.07.008 DOI: https://doi.org/10.1016/j.jpba.2014.07.008
13. Sohn JS, Choi JS. Development and evaluation of febuxostat solid dispersion through screening method. Saudi Pharm. 2023;31(9):101724. Available from: https://doi.org/10.1016/j.jsps.2023.101724 DOI: https://doi.org/10.1016/j.jsps.2023.101724
14. Aloisio C, G. de Oliveira A, Longhi M. Cyclodextrin and Meglumine-Based Microemulsions as a Poorly Water-Soluble Drug Delivery System. J Pharm Sci. 2016;105(9):2703–2711. http://dx.doi.org/10.1016/j.xphs.2015.11.045 DOI: https://doi.org/10.1016/j.xphs.2015.11.045
15. Furuishi T, Taguchi S, Wang S, Fukuzawa K, Yonemochi E. The Development and Characterization of Novel Ionic Liquids Based on Mono- and Dicarboxylates with Meglumine for Drug Solubilizers and Skin Permeation Enhancers. Pharmaceutics. 2024;16(3). https://doi.org/10.3390/pharmaceutics16030322 DOI: https://doi.org/10.3390/pharmaceutics16030322
16. Zaini E, Afriyani A, Fitriani L, Ismed F, Horikawa A. Improved Solubility and Dissolution Rates in Novel Multicomponent Crystals of Piperine with Succinic Acid. Sci Pharm. 2020;1–13. https://doi.org/10.3390/scipharm88020021 DOI: https://doi.org/10.3390/scipharm88020021
17. Fandaruff C, Vega-baudrit JR, Navarro-hoyos M, Lamas DG, Araya-sibaja AM. Saquinavir-Piperine Eutectic Mixture : Preparation, Characterization, and Dissolution Profile. Pharmaceutics. 2023;15:1–15. https://doi.org/10.3390/pharmaceutics15102446 DOI: https://doi.org/10.3390/pharmaceutics15102446
18. Czajkowska-Kośnik A, Misztalewska-Turkowicz I, Wilczewska AZ, Basa A, Winnicka K. Solid Dispersions Obtained by Ball Milling as Delivery Platform of Etodolac, a Model Poorly Soluble Drug. Materials (Basel). 2024;17(16). https://doi.org/10.3390/ma17163923 DOI: https://doi.org/10.3390/ma17163923
19. Erizal, Cahyati SY, Nurono SS, Halim A. Effect of milling on solid state transformation of sulfamethoxazole. Int J Pharmacol. 2008;4(2):140–144. http://dx.doi.org/10.3923/ijp.2008.140.144 DOI: https://doi.org/10.3923/ijp.2008.140.144
20. Christoforides E, Andreou A, Papaioannou A, Bethanis K. Structural Studies of Piperine Inclusion Complexes in Native and Derivative β-Cyclodextrins. Vol. 12, Biomolecules. 2022. p. 1–23. https://doi.org/10.3390/biom12121762 DOI: https://doi.org/10.3390/biom12121762
21. Bitay E, Gergely AL, Szabó ZI. Optimization and Production of Aceclofenac-Loaded Microfiber Solid Dispersion by Centrifugal Spinning. Pharmaceutics. 2023;15(9). https://doi.org/10.3390/pharmaceutics15092256 DOI: https://doi.org/10.3390/pharmaceutics15092256
22. Guo M, Sun X, Chen J, Cai T. Pharmaceutical Cocrystals: A Review of Preparations, Physicochemical Properties and Applications. Acta Pharm Sin B [Internet]. 2021;11(8):2537–2564. Available from: https://doi.org/10.1016/j.apsb.2021.03.030 DOI: https://doi.org/10.1016/j.apsb.2021.03.030
23. Kumar R, Thakur AK, Chaudhari P, Banerjee N. Particle Size Reduction Techniques of Pharmaceutical Compounds for the Enhancement of Their Dissolution Rate and Bioavailability. J Pharm Innov. 2022;17(2):333–352. https://doi.org/10.1007/s12247-020-09530-5 DOI: https://doi.org/10.1007/s12247-020-09530-5
24. Zaini E, Fitriani L, Sari RY, Rosaini H, Horikawa A, Uekusa H. Multicomponent Crystal of Mefenamic Acid and N-Methyl-D-Glucamine: Crystal Structures and Dissolution Study. J Pharm Sci. 2019;108(7):2341–2348. Available from: https://doi.org/10.1016/j.xphs.2019.02.003 DOI: https://doi.org/10.1016/j.xphs.2019.02.003
25. Zaini E, Wahyuni F, Salsabila H, Anggraini D, Yuliandra Y, Lucida H. Eutectic Mixture of Fenofibric Acid and Syringic Acid: Improvement of Dissolution Rate and Its Antihyperlipidemic Activity. ChemistrySelect. 2023;8(20):1–5. https://doi.org/10.1002/slct.202300044 DOI: https://doi.org/10.1002/slct.202300044
26. Liu Y, Yang F, Zhao X, Wang S, Yang Q, Zhang X. Crystal Structure, Solubility, and Pharmacokinetic Study on a Hesperetin Cocrystal with Piperine as Coformer. Pharmaceutics. 2022;14(1):1–14. https://doi.org/10.3390/pharmaceutics14010094 DOI: https://doi.org/10.3390/pharmaceutics14010094
27. Umar S, Putri N, Deni B, Erizal A. Multicomponent Crystal of Fenofibric Acid- Saccharin : Characterization and Antihyperlipidemic Effectiveness. Adv Heal Sci Res. 2021;40(Iccscp):104–109. https://doi.org/10.2991/ahsr.k.211105.015 DOI: https://doi.org/10.2991/ahsr.k.211105.015
28. Zaini E, Riska D, Oktavia MD, Ismed F, Fitriani L. Improving Dissolution Rate of Piperine by Multicomponent Crystal Formation with Saccharin. Res J Pharm Technol. 2020;13(April):1928–1932. https://doi.org/10.5958/0974-360X.2020.00347.9 DOI: https://doi.org/10.5958/0974-360X.2020.00347.9