Molecular docking analysis of 2,4,6-Octatrienoic acid with apoptotic protein targets: Insights into potential biological interactions
Main Article Content
Abstract
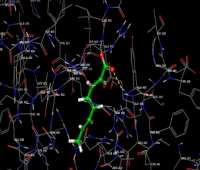
The discovery of novel compounds targeting apoptotic pathways presents significant potential for therapeutic advancements in cancer and other diseases marked by impaired apoptotic regulation. Dysregulation of apoptosis is a hallmark of various diseases, including cancer, neurodegenerative disorders, and autoimmune conditions. Therefore, identifying small molecules capable of modulating apoptotic pathways has gained considerable attention in drug discovery. In this study, the binding affinity and interaction dynamics of 2,4,6-Octatrienoic acid were evaluated through molecular docking with key apoptotic regulators, including pro-apoptotic, anti-apoptotic, and regulatory proteins. Docking simulations demonstrated strong binding affinities of 2,4,6-Octatrienoic acid to Caspase-7, BAX, Bcl-2-like protein 1, Bcl-2-like protein 2, Mcl-1, XIAP, and Apoptosis-inducing factor 1, with respective docking scores of -4.242, -3.427, -3.676, -2.917, -4.625, -3.007, and -3.593. These findings suggest its potential role in modulating apoptotic pathways. Interaction analyses revealed the presence of hydrogen bonding, hydrophobic interactions, and van der Waals forces, stabilizing these proteins in either their active or inhibited states. This suggests that 2,4,6-Octatrienoic acid may influence apoptosis by inducing programmed cell death in cancer cells or preventing excessive apoptosis in degenerative conditions. Overall, this study highlights 2,4,6-Octatrienoic acid as a promising candidate for apoptosis-targeted drug development. Future research should focus on detailed molecular dynamics simulations, structural modifications and preclinical evaluations to determine its efficacy in disease models. Further in vitro and in vivo studies are essential to validate these results and to explore the compound's therapeutic efficacy in greater depth.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Mustafa M, Ahmad R, Tantry IQ, Ahmad W, Siddiqui S, Alam M, Abbas K, Moinuddin, Hassan MI, Habib S, Islam S. Apoptosis: A comprehensive overview of signaling pathways, morphological changes, and physiological significance and therapeutic implications. Cells. 2024; 13(22):1-29
2. Engel N, Falodun A, Kühn J, Kragl U, Langer P, Nebe B. Pro-apoptotic and anti-adhesive effects of four African plant extracts on the breast cancer cell line MCF-7. BMC Complement Altern Med. 2014; 14(33)334-347
3. Karthik M, Paari E, Sivasankaran SM, Elanchezhiyan C, Manoharan S. Ursolic acid-loaded chitosan nanoparticles modulate the expression pattern of apoptotic markers towards oral tumour inhibition in golden Syrian hamsters. Trop J Nat Prod Res. 2023; 7(11):5250-5255.
4. Kristianingsih A, Reviono R, Wasita B. Molecular docking study of quercetin from ethanol extract of Mimosa pudica Linn on asthma biomarkers. Trop J Nat Prod Res. 2024; 8(10):8640-8645.
5. National Center for Biotechnology Information. PubChem compound summary for CID 5368831, 2,4,6-Octatrienoic acid. 2025. Available from: https://pubchemncbi.nlm.nih.gov/c ompound/Octa-2_4_6- trienoic-acid. Accessed Feb. 15, 2025.
6. Flori E, Mastrofrancesco A, Kovacs D, Ramot Y, Briganti S, Bellei B, Paus R, Picardo M. 2,4,6-Octatrienoic acid is a novel promoter of melanogenesis and antioxidant defence in normal human melanocytes via PPAR-γ activation. Pigment Cell Melanoma Res. 2011; 24(4):618-630.
7. Flori E, Mastrofrancesco A, Kovacs D, Bellei B, Briganti S, Maresca V, Cardinali G, Picardo M. The activation of PPARγ by 2,4,6-Octatrienoic acid protects human keratinocytes from UVR-induced damages. Sci Rep. 2017; 7(1):9241.
8. Pinzi L, Rastelli G. Molecular docking: Shifting paradigms in drug discovery. Int J Mol Sci. 2019; 20(18) 1-23
9. Spassov DS. Binding affinity determination in drug design: insights from lock and key, induced fit, conformational selection, and inhibitor trapping models. Int J Mol Sci. 2024; 25(13):1-13.
10. Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Res. 2000; 28(1):235-242.
11. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017; 7:4271, 1-13
12. Fu L, Shi S, Yi J, Wang N, He Y, Wu Z, Peng J, Deng Y, Wang W, Wu C, Lyu A, Zeng X, Zhao W, Hou T, Cao D. ADMETlab 3.0: An updated comprehensive online ADMET prediction platform enhanced with broader coverage, improved performance, API functionality and decision support. Nucleic Acids Res. 2024; 52(W1):W422-W431.
13. He R, Liu Y, Fu W, He X, Liu S, Xiao D, Tao Y. Mechanisms and cross-talk of regulated cell death and their epigenetic modifications in tumor progression. Mol Cancer. 2024; 23(1):1-54
14. Abaza A, Vasavada AM, Sadhu A, Valencia C, Fatima H, Nwankwo I, Anam M, Maharjan S, Amjad Z, Khan S. A systematic review of apoptosis in correlation with cancer: should apoptosis be the ultimate target for cancer treatment? Cureus. 2022; 14(8):e28496.
15. Caro-Gómez LA, López-Hidalgo M, Rosas-Trigueros JL, Torres-Rivera A, Vázquez-Hernández JJ, Benítez-Cardoza CG. Identification of compounds against anti-apoptotic bcl-2 protein using docking studies. ChemistrySelect. 2024; 9(1):e202302948.
16. Mohamad Rosdi MN, Mohd Arif S, Abu Bakar MH, Razali SA, Mohamed Zulkifli R, Ya'akob H. Molecular docking studies of bioactive compounds from Annona muricata Linn as potential inhibitors for Bcl-2, Bcl-w and Mcl-1 antiapoptotic proteins. Apoptosis. 2018; 23(1):27-40.
17. Nurhayati APD, Rihandoko A, Fadlan A, Ghaissani SS, Jadid N, Setiawan E. Anti-cancer potency by induced apoptosis by molecular docking P53, caspase, cyclin D1, cytotoxicity analysis and phagocytosis activity of trisindoline 1,3 and 4. Saudi Pharm J. 2022; 30(9):1345-1359.
18. Basumatary M, Talukdar A, Sharma M, Dutta A, Mukhopadhyay R, Doley R. Exploring the anticancer potential of Cytotoxin 10 from Naja kaouthia venom: Mechanistic insights from breast and lung cancer cell lines. Chem Biol Interact. 2024; 403:111254-111257