Phytochemical Profiling and Cytotoxicity Evaluation against HeLa Cell Line of the Extract of Artocarpus elasticus Stem Bark
Main Article Content
Abstract
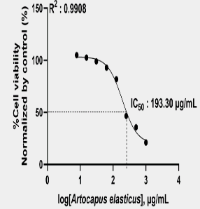
Cervical cancer is the second most common cancer affecting Indonesian women and is becoming a major health issue in Indonesia because of its high prevalence. The challenges associated with cancer treatment, such as adverse side effects and drug resistance, highlight the urgent need for new therapeutic options. Artocarpus is known for its diverse secondary metabolites, including flavonoids, which have shown potential anticancer properties. However, no research has examined the potential of Artocarpus elasticus as an anticervical cancer agent and the compounds responsible for anticervical cancer activity. Therefore, the objectives of this study were to evaluate the cytotoxic activity of the A. elasticus stem bark extract against the HeLa cell line and to identify the secondary metabolites of the active extract. The extract was obtained by the ultrasound-assisted extraction method using 96% ethanol. The cytotoxicity of the extract was evaluated by the PrestoBlue assay, and the data were analyzed by the linear regression method using Microsoft Excel 2016. The secondary metabolites contained in the extract were identified using UHPLC-Q-Orbitrap HRMS analysis. Results showed that the extract exhibited moderate cytotoxic activity against the HeLa cell line with an IC50 of 193.30 µg/mL. A total of 13 metabolites were identified in the extract, including prenylated flavonoids, such as artonin E and artocarpin. Thus, the A. elasticus stem bark extract has moderate cytotoxic activity against the HeLa cell line and contains a bioactive compound that has the potential to be further investigated as a candidate anticervical cancer agent.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71(3):209-249.
Ministry of Health Republic of Indonesia. National Cervical Cancer Elimination Plan for Indonesia 2023-2030. [Online] 2023 [Accessed 2024 April
27] Available from: https://kemkes.go.id/eng/media/subfolder/pedoman/national-cervical-cancer-elimination-plan-for-indonesia-2023-2030
Hong Y, Yuhan L, Youhui G, Zhanying W, Shili Z, Xiaoting H, Wenhua Y. Death anxiety among advanced cancer patients: a cross-sectional survey. Support Care Cancer. 2022; 30(4):3531-3539.
Aslam MS, Naveed S, Ahmed A, Abbas Z, Gull I, Athar MA. Side Effects of Chemotherapy in Cancer Patients and Evaluation of Patients Opinion about Starvation Based Differential Chemotherapy. J Cancer Ther. 2014; 5(8):817-822.
Adumuah NN, Quarshie JT, Danwonno H, Aikins AR, Ametefe EN. Exploring Anti-Breast Cancer Effects of Live Pediococcus acidilactici and Its Cell-Free Supernatant Isolated from Human Breast Milk. Int J Breast Cancer. 2024; 2024:1841909
Lin SR, Chang CH, Hsu CF, Tsai MJ, Cheng H, Leong MK, Sung PJ, Chen JC, Weng CF. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br J Pharmacol. 2020; 177(6):1409-1423.
Okafor CE, Ijoma IK, Igboamalu CA, Ezebalu CE, Eze CF, Osita-Chikeze JC, Uzor CE, Ekwuekwe AL. Secondary metabolites, spectra characterization, and antioxidant correlation analysis of the polar and nonpolar extracts of Bryophyllum pinnatum (Lam) Oken. BioTechnologia (Pozn). 2024; 105(2):121-136.
Newman DJ, Cragg GM. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J Nat Prod. 2020; 83(3):770-803.
Soifoini T, Donno D, Jeannoda V, Rakoto DD, Msahazi A, Farhat SMM, Oulam MZ, Beccaro GL. Phytochemical composition, antibacterial activity, and antioxidant properties of the Artocarpus altilis fruits to promote their consumption in the Comoros islands as potential health-promoting food or a source of bioactive molecules for the food industry. Foods. 2021; 10(9):2136.
Etti IC, Abdullah R, Kadir A, Hashim NM, Yeap SK, Imam MU, Ramli F, Malami I, Lam KL, Etti U, Waziri P, Rahman M. The molecular mechanism of the anticancer effect of Artonin E in MDA-MB 231 triple negative breast cancer cells. PLoS One. 2017; 12(8):e0182357.
Rahman MA, Ramli F, Karimian H, Dehghan F, Nordin N, Ali HM, Mohan S, Hashim NM. Artonin E induces apoptosis via mitochondrial dysregulation in SKOV-3 ovarian cancer cells. PLoS One. 2016; 11(3):e0151466.
Baiseitova A, Shah AB, Khan AM, Idrees M, Kim JH, Lee YH, Kong I-K, Park KH. Antioxidant potentials of furanodihydrobenzoxanthones from Artocarpus elasticus and their protection against oxLDL induced injury in SH-SY5Y cells. Biomed Pharmacother. 2023; 165:115278.
Hakim EH, Achmad SA, Juliawaty LD, Makmur L, Syah YM, Aimi N, Kitajima M, Ghisalberti EL. Prenylated flavonoids and related compounds of the Indonesian Artocarpus (Moraceae). J Nat Med. 2006; 60(3):161-184.
Jayasinghe ULB, Samarakoon TB, Kumarihamy BMM, Hara N, Fujimoto Y. Four new prenylated flavonoids and xanthones from root bark of Artocarpus nobilis. Fitoterapia. 2008; 79(1):37-41.
Lv HW, Wang QL, Luo M, Zhu MD, Liang HM, Li WJ, Cai H, Zhou ZB, Wang H, Tong SQ, Li XN. Phytochemistry and pharmacology of natural prenylated flavonoids. Arch Pharm Res. 2023; 46(4):207-272.
Ersam T, Achmad SA, Ghisalberti EL, Hakim EH, Makmur L, Syah YM. A new isoprenylated chalcone, artoindonesianin J, from the root and tree bark of Artocarpus bracteate. J. Chem. Research. 2002; 2002(4):186-187.
Chan EWC, Wong SK, Tangah J, Chan HT. Chemistry and Pharmacology of Artocarpin: An isoprenyl flavone from Artocarpus spesies. Sys Rev Pharm. 2018; 9(1):58-63.
Ye JB, Ren G, Li WY, Zhong GY, Zhang M, Yuan JB, Lu T. Characterization and identification of prenylated flavonoids from Artocarpus heterophyllus Lam. roots by Quadrupole Time-of-Flight and Linear Trap Quadrupole Orbitrap Mass Spectrometry. Molecules. 2019; 24(24):4591.
Mustapha I, Juliawaty LD, Syah YM, Hakim EH, Latip J, Ghisalberti EL. An oxepinoflavone from Artocarpus elasticus with cytotoxic activity against P-388 Cells. Arch Pharm Res. 2009; 32(2):191-194.
Wang YH, Hou AJ, Chen L, Chen DF, Sun HD, Zhao QS, Bastow KF, Nakanish Y, Wang XH, Lee KH. New isoprenylated flavones, artochamins A−E, and cytotoxic principles from Artocarpus chama. J Nat Prod. 2004; 67(5):757-761.
Jagtap UB, Bapat VA. Artocarpus: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2010; 129(2):142-166.
Bailly C. Anticancer mechanism of artonin E and related prenylated flavonoids from the medicinal plant Artocarpus elasticus. Asian J Nat Prod Biochem. 2021; 19(2):45-57.
Kijjoa A, Cidade H, Pinto M, Gonzalez MJTG, Anantachoke C, Gedris TE, Herz W. Prenylflavonoids from Artocarpus elasticus. Phytochemistry. 1996; 43(3):691-694.
Cidade HM, Nacimento MS, Pinto MM, Kijjoa A, Silva AM, Herz W. Artelastocarpin and carpelastofuran, two new flavones, and cytotoxicities of prenyl flavonoids from Artocarpus elasticus against three cancer cell lines. Planta Med. 2001; 67(9):867-870.
Ramli F, Rahmani M, Ismail IS, Sukari MA, Rahman MA, Zajmi A, Akim AM, Hashim NM, Go R. A new bioactive secondary metabolite from Artocarpus elasticus. Nat Prod Commun. 2016; 11(8):1103-1106.
Kurang RY, Ersam T. Prenylated flavone of the bark of Artocarpus elasticus from Alor Island-NTT Indonesia. J Applied Chem Sci. 2017; 4:322-324.
Fajriah S, Megawati M, Darmawan A. Cytotoxic Activity of Two Compounds Extracted from Artocarpus elasticus Reinw. Ex Blume Leaves on Selected Cancer Cell Lines.Trop J Nat Prod Res. 2021; 5(10):1724-1726.
Srisawat T, Chumkaew P, Heed-Chim W, Sukpondma Y, Kanokwiroon K. Phytochemical screening and cytotoxicity of crude extracts of Vatica
diospyroides symington type LS. Trop J Pharm Res. 2013; 12(1):71-76.