α-Glucosidase and xanthine oxidase inhibitory activities from the fruits of Thai Averrhoa bilimbi L.
Main Article Content
Abstract
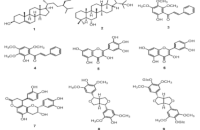
Averrhoa bilimbi L., a tropical plant native to Southeast Asia, is traditionally used in folk medicine to treat various ailments, including infections, inflammation, hypertension, obesity, and diabetes. In this study, the fruits of A. bilimbi L. were successfully isolated and identified as β-sitosterol (1), zeorin (2), helilandin B (3), 2'-hydroxy-3',4',6'-trimethoxychalcone (4), myricetin (5), quercetin (6), cinchonain Ia (7), syringaresinol (8), and syringaresinol diglucoside (9). All the isolated compounds were tested for their inhibitory effects on α-glucosidase and xanthine oxidase (XO) activities. Compounds 3 and 4 demonstrated the strongest α-glucosidase inhibitory activity, with IC50 values of 4.10 ± 0.20 and 4.79 ± 0.27 µM, respectively. Compound 4 exhibited the highest XO inhibition, with an IC50 value of 48.4 ± 0.03 µM. These findings highlight the potential of the bioactive compounds from A. bilimbi L. as promising candidates for therapeutic applications in the management of diabetes and hyperuricemia.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
1. Alhassan AM, Ahmed QU. Averrhoa bilimbi Linn.: A review of its ethnomedicinal uses, phytochemistry, and pharmacology. J Pharm Bioallied Sci. 2016; 8(4): 265–271.
2. Prastiyanto ME, Wardoyo FA, Wilson W, Darmawati S. Antibacterial activity of various extracts of Averrhoa bilimbi against multidrug-resistant bacteria. Biosaintifika. 2020; 12(2): 163–168.
3. Kumar KA, Gousia SK, Anupama M, Latha JNL. A review on phytochemical constituents and biological assays of Averrhoa bilimbi. Int J Pharm Pharm Sci Res. 2013; 3(4): 136–139.
4. Garg M, Chaudhary SK, Kumari S, Goyal A. Phytochemical, biological, and traditional claims on Averrhoa bilimbi: An overview. Indian J Pharm Sci. 2022; 84(3): 532–542.
5. Ahmed QU, Alhassan AM, Khatib A, Shah SAA, Hasan MM, Sarian MN. Antiradical and xanthine oxidase inhibitory activity evaluations of Averrhoa bilimbi L. leaves and tentative identification of bioactive constituents through LC-QTOF-MS/MS and molecular docking approach. Antioxidants. 2018; 7(10): 137.
6. Miraj AJ, Kabir A, Mamun Y, Akhter S, Ahammed MS, Sultana S, et al. Evaluation of the analgesic and anti-inflammatory activities of methanolic extracts of the leaves of Averrhoa bilimbi. Discov Phytomed. 2019; 6(1): 12.
7. Santi TD, Siregar TN, Sutriana A, Andini R, Candra A. Wound healing activity of transdermal patches of Carica papaya, Chromolaena odorata, and Averrhoa bilimbi leaves on incision wounds of hyperglycemic rats. Trends Sci. 2023; 20(12): 6944.
8. Sarker MAM, Chowdhury ASFU. Analgesic effect of methanolic extracts of leaf, bark, and fruit of Averrhoa bilimbi Linn. Bangladesh Med Res Counc Bull. 2022; 48(2): 120–126.
9. Meilina R, Suwarso EDY, Dalimunthe A. Relaxation effect of ethanolic extract of Averrhoa bilimbi L. leaves on ileum smooth muscle contraction of in vitro isolated rat (Rattus norvegicus). Asian J Pharm Clin Res. 2018; 11:135–137.
10. Prabhu R, Fernandes R, Govinda KA. Phytoconstituents isolation and hepatoprotective activity potential of Averrhoa bilimbi leaf extract. J Pharm Res Int. 2021; 33(58B): 573–581.
11. Bipat R, Toelsie JR, Joemmanbaks RF, Gummels JM, Klaverweide J, Jhanjan N, et al. Effects of plants popularly used against hypertension on norepinephrine-stimulated guinea pig atria. Pharmacogn Mag. 2008; 4(13): 12.
12. Nair MS, Soren K, Singh V, Boro B. Anticancer activity of fruit and leaf extracts of Averrhoa bilimbi on MCF-7 human breast cancer cell lines: A preliminary study. Austin J Pharmacol Ther. 2016; 4(2): 1082.
13. Hoang LTTT, Dong PSN, Nguyen VK, Thao VTM, Ramadhan R, Jutakanoke R, Sichaem J. β-amyrin heptadecanoate, a new oleanane triterpenoid with α-glucosidase inhibitory and cytotoxic activities from the leaves of Averrhoa bilimbi L. Nat Prod Res. 2024. Doi: 10.1080/14786419.2024.2425045
14. Azeem AK, Vrushabendraswami BM. Hypolipidemic evaluation of Averrhoa bilimbi leaf ethanolic extracts on streptozotocin-induced diabetic rats. J Innov Pharm Biol Sci. 2015; 2(4): 649–652.
15. Gunawan C, Cordero A, Paano A. Structure elucidation of two new phytol derivatives, a new phenolic compound, and other metabolites of Averrhoa bilimbi. Res Congr. 2013 Mar; 7–9.
16. Auw L, Subehan, Sukrasno, Kadota S, Tezuka Y. Constituents of Indonesian medicinal plant Averrhoa bilimbi and their cytochrome P450 3A4 and 2D6 inhibitory activities. Nat Prod Commun. 2015; 10(1): 57–62.
17. Rohman F, Putra WE, Sustiprijatno, Widiastuti D. Virtual assessment of Imperata cylindrica roots bioactive compounds as a potential inhibitor for alpha-glucosidase: The study of Tengger tribe's medicinal plant. Trop J Nat Prod Res. 2021; 5(7): 1240–1245.
18. Osiako FH, Samuel BB, Oluyemi WM. Effects of selected Terminalia and Ficus species in the inhibition of α-amylase and α-glucosidase enzymes. Trop J Nat Prod Res. 2023; 7(8): 3775–3780.
19. Li Z, Wang H, Sun S, Shao Z, Lv C, Dong X, Wang W. Ellagitannins from pomegranate (Punica granatum L.) flower with xanthine oxidase and α-glucosidase inhibitory activities. J Funct Foods. 2024; 116: 106153.
20. Wang H, Zhou X, Liu Y, Xie W, Yang D, Huo D, Wang R. Identification and molecular docking of xanthine oxidase and α‐glucosidase inhibitors in Opuntia ficus‐indica fruit. J Food Sci. 2024; 89(7): 4192–4204.
21. Rattanapan J, Sichaem J, Tip-pyang S. Chemical constituents and antioxidant activity from the stems of Alyxia reinwardtii. Rec Nat Prod. 2012; 6(3): 288–291.
22. Sichaem J, Aree T, Lugsanangarm K, Tip-Pyang S. Identification of highly potent α-glucosidase inhibitory and antioxidant constituents from Zizyphus rugosa bark: Enzyme kinetic and molecular docking studies with active metabolites. Pharm Biol. 2017; 55(1): 1436–1441.
23. Hang DTT, Trang DT, Dung DT, Yen DTH, Hoang NH, Bang NA, Cuc NT, Nhiem NX, Huong PTT, Tai BH, Kiem PV. Guaianolide sesquiterpenes and benzoate esters from the aerial parts of Siegesbeckia orientalis L. and their xanthine oxidase inhibitory activity. Phytochemistry 2021; 190: 112889.
24. Chaturvedula VSP, Prakash I. Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus. Int Curr Pharm J. 2012; 1(9): 239–242.
25. Yosioka I, Nakanishi T, Yamauchi H, Kitagawa I. Revised structure of zeorin and its correlation with leucotylin. Tetrahedron Lett. 1971; 12(16): 1161–1164.
26. Ichino K, Tanaka H, Ito K, Tanaka T, Mizuno M. Synthesis of helilandin B, pashanone, and their isomers. J Nat Prod. 1988; 51(5): 906–914.
27. Lien TP, Porzel A, Schmidt J, Van Sung T, Adam G. Chalconoids from Fissistigma bracteolatum. Phytochemistry. 2000; 53(8): 991–995.
28. Banerjee C, Nandy S, Chakraborty J, Kumar D. Myricitrin–a flavonoid isolated from the Indian olive tree (Elaeocarpus floribundus)–inhibits Monoamine oxidase in the brain and elevates striatal dopamine levels: therapeutic implications against Parkinson’s disease. Food Funct. 2022; 13(12): 6545–6559.
29. Sinha R, Gadhwal MK, Joshi UJ, Srivastava S, Govil G. Modifying effect of quercetin on model biomembranes: studied by molecular dynamic simulation, DSC and NMR. Int J Curr Pharm Res. 2012; 4(1): 70–79.
30. Resende FO, Rodrigues-Filho E, Luftmann H, Petereit F, Mello JC. Phenylpropanoid substituted flavan-3-ols from Trichilia catigua and their in vitro antioxidative activity. J Braz Chem Soc. 2011; 22: 2087–2093.
31. Gohari AR, Saeidnia S, Bayati-Moghadam M, Amin G. Lignans and neolignans from Stelleropsis antoninae. Daru. 2011; 19(1): 74.
32. Wang Z, Zhang L, Sun Y. Semipreparative separation and determination of eleutheroside E in Acanthopanax giraldii Harms by high-performance liquid chromatography. J Chromatogr Sci. 2005; 43(5): 249–252.
33. Cai CY, Rao L, Rao Y, Guo JX, Xiao ZZ, Cao JY, Wang B. Analogues of xanthones—Chalcones and bis-chalcones as α-glucosidase inhibitors and anti-diabetes candidates. Eur J Med Chem. 2017; 130: 51–59.
34. Tran TD, Tu VL, Hoang TM, Dat TV, Tam DNH, Phat NT, Hung DT, Huynh HH, Do TC, Le HH, Minh LHN. A review of the in vitro inhibition of α-amylase and α-glucosidase by chalcone derivatives. Cureus. 2023; 15(4): e37267.
35. Kuroda M, Iwabuchi K, Usui S, Akiyama N, Mimaki Y. Chemical compounds from the leaves of Verbascum thapsus and their xanthine oxidase inhibitory activity. Shoyakugaku Zasshi. 2017; 71(1): 49–50.
36. Alizadeh N, Eskandani M, Tondro K, Rashidi MR, Nazemiyeh H. Inhibitory effects of flavonolignans from Silybum marianum (L.) Gaertn (milk thistle) on function of aldehyde oxidase and xanthine oxidase in rats. Lett Drug Des Discov. 2018; 15(3): 256–262.