Synthesis of a Novel Chemical J5 brown Dye for Staining Specific Bone Histological Sections in Green Swordtail Fish (Xiphophorus hellerii)
Main Article Content
Abstract
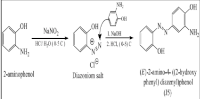
The creation of aromatic pigments in 1859 revolutionized the dye industry, with azo dyes emerging as the most significant class due to their versatility, cost-effectiveness, and widespread applications in textiles, food coloring, and medical fields. The present study aimed to synthesize and evaluate J5 brown, a novel azo dye, for staining specific bone histological sections in green swordtail fish (Xiphophorus hellerii). The dye was synthesized using the diazotization and coupling reaction. Nuclear magnetic resonance (NMR) spectrometry, infrared spectrometry, and mass spectrometry (MS) were used to confirm the structure of the dye. Histological sections of the fish samples were prepared, and the dye was evaluated for its efficacy in staining fish bone tissues. The synthesized J5 brown resulted in a brown powder with a yield of 87% and a melting point of 162–164°C. When the dye's absorption properties were evaluated over a range of pH values (2–12), the results revealed maximum absorption at 430 nm between pH 2 and 8 and at 350 nm between pH 9 and 12. Solvent polarity also influenced the dye's absorption, with notable redshifts observed in polar solvents and water. J5 brown dye effectively stained fish bone tissues, outperforming Alizarin Red in highlighting bone structures with approximately 80% accuracy compared to traditional bone stains. The present study’s findings revealed that the novel dye offers a cost-effective, stable alternative for bone tissue staining, with potential applications in both clinical and research settings. Further studies will explore its suitability for staining other tissues and its toxicity profile.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
1. Christie RM. Colour: A brief historical perspective. In: Colour Chem. ; 2007:1-11.
2. Khanum R, Shoukat Ali RA, Rangaswamy HR, Santhosh Kumar SR, Prashantha AG, Jagadisha AS. Recent review on Synthesis, spectral Studies, versatile applications of azo dyes and its metal complexes. Results Chem. 2023;5:100890.
3. Benkhaya S, M’rabet S, El Harfi A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon. 2020;6(1):e03271.
4. Locher C.P, Burch M.T, Mower H. F, Berestecky J, Davis H, Van Poel B, Lasure A, Vanden Berghe D. A, Vlietinck A. J. Anti-microbial activity and anti-complement activity of extracts obtained from selected Hawaiian medicinal plants. J Ethnopharmacol. 1995;49(1):23-32.
5. Khan MN, Parmar DK, Das D. Recent Applications of Azo Dyes: A Paradigm Shift from Medicinal Chemistry to Biomedical Sciences. Mini-Reviews Med Chem. 2021;21(9):1071-1084.
6. Majeed H. Synthesis , Characterization , and study of the Spectral and Electronic Properties of a New Azo Dyes Compounds. Univ Thi-Qar J Sci. 2019;4(1):91-101.
7. Ahlström LH, Sparr Eskilsson C, Björklund E. Determination of banned azo dyes in consumer goods. TrAC Trends Anal Chem. 2005;24(1):49-56.
8. Kunrath MF, Hübler R. A bone preservation protocol that enables evaluation of osseointegration of implants with micro- and nanotextured surfaces. Biotech Histochem. 2019;94(4):261-270.
9. Russell DK, Crabtree WN, Gill GW. Cytopreparatory Techniques. In: The Paris System for Reporting Urinary Cytology. Springer International Publishing; 2022:221-247.
10. Atkins P, Paula J de, Keeler J. Atkins’ Physical Chemistry. Oxford University Press; 2022.
11. Clarke B. Normal Bone Anatomy and Physiology. Clin J Am Soc Nephrol. 2008;3(Supplement_3):S131-S139.
12. Mohammed LA, Mahdi NI, Aldujaili RAB. Preparation ,Characterization and The Biological Activity Study of A new heterocyclic (Azo-Schiff base) ligand and Their Complexation with {Co,Ni,Cu,Zn(II)}Ions. Egypt J Chem. 2020;63(1):289-300.
13. Fahad T, Ali A, Fahad TA, Ali AA, Baty AH. synthesis, characterization and analytical studies of some new azodyes driven from o-vanillin. World J Pharm Res. 2019;8
14. Burstone MS. new histochemical techniques for the demonstration of tissue oxidase (cytochrome oxidase). J Histochem Cytochem. 2014;7(2):112-122.
15. Hamsa H. A, Faeza A. A, Aqeel T. K, Mohammed K. M, Kawkab A. H, Ahmed M. J. Synthesis, Characterization, Cytotoxic Evaluation on MCF-7 Breast Cancer Cells, and Theoretical Studies of Novel 1,2,3-Triazoles. Trop J Nat Prod Res. 2023; 7(7):3306-3313
16. Tanaka K, Padermpole K, Hisanaga T. Photocatalytic degradation of commercial azo dyes. Water Res. 2000;34(1):327-333.
17. Al-Muhsin AA, Fahad TA, Ali AA. Preparation and characterization azo dyes derived from 4- hydroxycoumarin and studying their analytical Applications. J Phys Conf Ser. 2021;1999(1):012010.
18. Stalin T, Rajendiran N. Intramolecular charge transfer effects on 3-aminobenzoic acid. Chem Phys. 2006;322(3):311-322.
19. Al-Waeli JH, AbdullMajed HA, Ismael SM. Studies of spectroanalytical and the biological effectiveness of the Azo compound (J25) prepared from Ethyl p-aminobenzoate. Basrah Res Sci. 2024;50(1):11.
20. Airinei A, Homocianu M, Dorohoi DO. Changes induced by solvent polarity in electronic absorption spectra of some azo disperse dyes. J Mol Liq. 2010;157(1):13-17.
21. Jian Y, Tian X, Li Q, Li B, Peng Z. Comparison of methods for staining microvessels in bone. Biotech Histochem. 2012;87(6):428-431.
22. Burstone MS. new histochemical techniques for the demonstration of tissue oxidase (cytochrome oxidase). J Histochem Cytochem. 1959;7(2):112-122.
23. Aqeil AT. Anthocyanin pigment is similar to chemical dyes. 2021;0(2020):1-7.
24. Puchtler H, Meloan SN, Terry MS. on the history and mechanism of alizarin and alizarin red s stains for calcium. J Histochem Cytochem. 1969;17(2):110-124.