Morpho-physiological parameters of Cedrus atlantica Manetti pollen discriminate genetically distinct populations of climatically different regions in the Moroccan Atlas
Main Article Content
Abstract
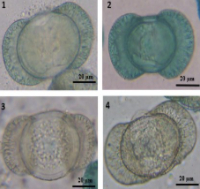
In North Africa’s forests of Cedrus atlantica many tree stands perished in the last 130 years due to environmental changes. Variations in some morpho-physiological characters of cedar pollens were studied in three populations localized in Azrou and Midelt regions of Moroccan Atlas having different local environmental conditions and levels of genetic diversity. Results of Spearman nonparametric coefficient of correlation showed significant correlations ( -0.726 ≤ r ≤ 0.812) (p < 0.01). Corpus length (Lc) and volume (Vc), balloon volume (Vb) and the volume total of pollen (Vtp) were negatively related to annual mean precipitation (P) and positively to longitude (Long) and altitude (Alt). Lc and Vc were also negatively correlated with latitude (Lat) while positively with annual mean temperature (T) for Vc. Balloon height (Hb), number of germinated pollen (NGP) and germination frequency of pollen (GFP) were positively linked to Alt. Pollen starch content (Starch) was negatively correlated to T and Long and positively to P and Lat. Production of pollen (NTP) was positively correlated with P and negatively with Long and Alt. The PCA analysis showed that Lc, Vc, Lg, Hb, Vb, Vtp, NGP, GFP, NTP were influenced by climatic conditions and geographic location. The ascending hierarchical classification showed that populations of Ait Oufella and Ait Ayach are separated from the Seheb population. Tukey’s test showed that at least five morpho-physiological characters separated significantly the populations. The structuration of morphological traits of Atlas cedar pollen of the populations was discussed in relation to physiological traits and local environmental conditions.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Hallé F, Oldeman RAA. Essay on the architecture and growth dynamics of tropical trees. Masson, Paris Collection of Monographs of Botany and Plant Biology. 1970; 6.
Mortensen LM, Ulsaker R. Effect of CO2 concentration and light levels on growth, flowering and photosynthesis of Begonia × hiemalis Fotsch. Scientia Horticulturae. 1985; 27(1–2) :133-141. https://doi.org/10.1016/0304-4238(85)90063-9
Nuñez CI, Nuñez MA, Kitzberger T. Sex-related spatial segregation and growth in a dioecious conifer along environmental gradients in northwestern Patagonia. Écoscience. 2008; 15: 73-80. https://doi.org/10.2980/1195-6860(2008)15[73:SSSAGI]2.0.CO;2
Piola F, Label P, Vergne P, von Aderkas P, Rohr R. Effects of endogenous ABA levels and temperature on cedar (Cedrus libani Loudon) bud dormancy in vitro. Plant Cell Reports. 1998; 18: 279-283. https://doi.org/10.1007/s002990050571
Wahid N, Gonzales-Martinez SC, El Hadrami I, Boulli A. Variation of morphological traits in natural populations of maritime pine (Pinus pinaster Ait) in Morocco. Ann. For. Sci. 2006; 63 (1): 83-92. https://doi.org/10.1051/forest:20050100
Caliskan B, Colgecen H, Pehlivan S. Pollen characteristics and in vitro pollen germination of Cedrus libani A. Rich. African Journal of Biotechnology. 2009; 8(21): 5696-5701. https://doi.org/10.5897/AJB09.663
Breygina M, Klimenko E, Shilov E, Podolyan A, Mamaeva A, Zgoda V, Fesenko I. Hydrogen peroxide in tobacco stigma exudate affects pollen proteome and membrane potential in pollen tubes. Plant Biol 2021; 23: 592–602. https://doi.org/10.1111/plb.13255
Senghor C, Saouab F-E, Bendriss Amraoui M. Pollen indices of Cedrus atlantica Manetti populations vary with geographical localities in the Moroccan Atlas Mountains. Woods & Forests of the Tropics. 2024; 359: 69-83. https://doi.org/10.19182/bft2024.359.a37273
Tejaswini N. Variability of pollen grain features: a plant strategy to maximize reproductive fitness in two species of Dianthus?. Sex Plant Reprod . 2002; 14: 347-353. https://doi.org/10.1007/s00497-002-0130-z
Osborn JM, El-Ghazaly G, Cooper RL. Development of the exineless pollen wall in Callitriche truncate (Callitrichaceae) and the evolution of underwater pollination. Plant Syst. Evol . 2001; 228 (1) :81-87. https://doi.org/10.1007/s006060170039
Ćalić D, Devrnja N, Kostić I, Kostić M. Pollen morphology, viability, and germination of Prunus domestica cv. Požegača. Scientia Horticulturae. 2013; 155: 118-122. https://doi.org/10.1016/j.scienta.2013.03.017
Schoch-Bodmer H. Zur Methodik der Grossenbestimmung von Pollenkorner, mit besonderer Berucksichtigung von Corylus avellana. Berichte Schweizerische Botanische Gesellschaft. 1936; 45: 62-70.
Kurtz EB, Liverman JL, Tucker H. Some effects of temperature on pollen characters. Bulletin of the Torrey Botanical Club. 1958; 87: 85-94.
El Bakkali N, Bendriss Amraoui M. Structure of Needle Highlights Ecological Adaptability and Microevolution of Natural Populations of Cedrus atlantica in Morocco. International Journal of Forestry Research. 2022b; 2022: 1-9. https://doi.org/10.1155/2022/5415807
Benabid A. Biogeography, phytosociology and phytodynamics of Atlas cedar forests Cedrus atlantica (Manetti). Ann. Rech. For. Maroc .1994; 27: 61-76.
Terrab A, Paun O, Talavera S, Tremetsberger K, Arista M, Stuessy TF. Genetic diversity and population structure in natural populations of Moroccan Atlas cedar (Cedrus atlantica; Pinaceae) determined with cpSSR markers. Am. J. Bot . 2006; 93:1274–1280. https://doi.org/10.3732/ajb.93.9.1274
Messaoudène M, Loukkas A, Janin G, Tafer M, Dilem A, Gonçalez J. Physical properties of cedar thinning wood (Cedrus atlantica), containing compression wood, from the Djurdjura Atlas (Algeria). Ann. For. Sci. 2004; 61 (6) : 589-595. https://hal.science/hal-00883791
Aoubouazza M. Estimation of water needs of the Cedar in Ras El Ma and Boutrouba (Central Tabular Middle Atlas). Rev. Mar. Sci. Agron. Vét. 2018 ; 6 (1) : 36-47. https://agrimaroc.org/index.php/Actes_IAVH2/article/view/508
Karam MJ, Aouad M, Roig A, Bile A, Bou Dagher-Kharrat M, Klein EK, Fady B, Lefèvre F. Characterizing the genetic diversity of Atlas cedar and phylogeny of Mediterranean Cedrus species with a new multiplex of 16 SSR markers. Tree Genetics & Genomes. 2019, 15: 60. https://doi.org/10.1007/s11295-019-1366-1
Cheddadi R, Fady B, Francois L, Hajar L, Suc JP, Huang K, Demarteau M, Vendramin GG, Ortu E. Putative glacial refugia of Cedrus atlantica deduced from Quaternary pollen records and modern genetic diversity. Journal of Biogeography. 2009; 36: 1361-1371. https://doi.org/10.1111/j.1365-2699.2008.02063.x
El Bakkali N, Bendriss Amraoui M. The length, number, and endodermis area of needles discriminate two genetically distinct populations of Cedrus atlantica Manetti in the Moroccan Middle Atlas. Acta Soc Bot Pol. 2018; 87(3):3591.
Derridj A, Cadeac F, Durrieu G. Study of the geographical variability of the pollen dimensions of the Atlas cedar (Cedrus atlantica Manetti) in Algeria, Bulletin of the Botanical Society of France. Botanical Letters.1991 ;138(3): 215-230. https://doi.org/10.1080/01811797.1991.10824923
Bell BA, Bishop TH, Fletcher WJ, Ryan P, Ilmen R. Cedrus atlantica pollen morphology and investigation of grain size variability using laser diffraction granulometry. Palynology. 2018; 42 :339-353. https://doi.org/10.1080/01916122.2017.1356760
Rhanem M. Outline of a geomorphological typology of some cedar forests with Cedrus atlantica Man. in the eastern High Atlas of Midelt (Morocco). Threats and prospects for conservation, management and restoration. Quad. Bot. Amb. Appl. 2010, 21: 135-153. http://acrinwafrica.mnhn.fr/SiteAcri/pdf_biblio/Rhanem_M_2010.pdf
Rhanem M. Aridification of the regional climate and rise of the lower limit of the Atlas cedar (Cedrus atlantica Manetti) at the edge of the Midelt plain (Morocco). Physio-Geo Physical geography and environment. 2011 ; 5 :143-165. https://doi.org/10.4000/physio-geo.1983
Roy K, Balch DP, Hellberg ME. Spatial patterns of morphological diversity across the Indo-Pacific: Analyses using stromboid gastropods. Proc. R. Soc. Lond. B . 2001; 268: 2503–2508. https://doi.org/10.1098/rspb.2000.1428
Pither J. Climate tolerance and interspecific variation in geographic range size. Proceeding of the Royal Society of London. Series B. Biological Sciences. 2003; 270: 475-481. https://doi.org/10.1098/rspb.2002.2275
Svenning JC. Deterministic Plio-Pleistocene extinctions in the European cool-temperate tree flora. Ecology Letters. 2003; 6: 646-653. https://doi.org/10.1046/j.1461-0248.2003.00477.x
Terrab A, Hampe A, Lepais O, Talavera S, Vela E, Stuessy TF. Phylogeography of North African Atlas cedar (Cedrus atlantica, Pinaceae): Combined molecular and fossil data reveal a complex Quaternary history. Am. J. Bot . 2008; 95(10) :1262-1269. https://doi.org/10.3732/ajb.0800010
Bouahmed A, Vessella F, Schirone B, Krouchi F, Derridj A. Modeling Cedrus atlantica potential distribution in North Africa across time: new putative glacial refugia and future range shifts under climate change. Regional Environmental Change. 2019; 19: 1667-1682. https://doi.org/10.1007/s10113-019-01503-w
Zinn KE, Tunc-Ozdemir M, Harper JF. Temperature stress and plant sexual reproduction: uncovering the weakest links. Journal of Experimental Botany. 2010; 61(7):1959-1968. https://doi.org/10.1093/jxb/erq053
Mehmood M, Qamar R, Joyia FA. Effect of High Temperature Stress on Pollen Grains in Sunflower (Helianthus annuus L.) Inbred Lines. Braz. arch. biol. Technol . 2023; 66. https://doi.org/10.1590/1678-4324-2023220927
Elena B. Aspects of the pollen grains diameter variability and the pollen viability to some sunflower genotypes. Journal of Horticulture and Forestry. 2013; 17(1):161-165.
Lloret F, Keeling EG, Sala A. Components of tree resilience: effects of successive low-growth episodes in old ponderosa pine forests. Oikos. 2011; 120: 1909-1920.
Alberto FJ, Aitken SN, Alia R, Gonzalez-Martinez SC, Hanninen H, Kremer A, Lefèvre F, Lenormand T, Yeaman S, Whetten R, Savolainen O. Potential for evolutionary responses to climate change evidence from tree populations. Glob Change Biol. 2013; 19: 1645–1661. https://doi.org/10.1111/gcb.12181
Mencuccini M, Minunno F, Salmon Y, Martı́nez-Vilalta J, Hölttä T. Coordination of physiological traits involved in drought-induced mortality of woody plants. New Phytol . 2015; 208: 96-409. https://doi.org/10.1111/nph.13461
Nicholić B, Bojović S, Marin PD. Morpho-anatomical properties of Pinus heldreichii needles from natural populations in Montenegro and Serbia. Plant Biosystems. 2016; 150 (2): 254–263. https://doi.org/10.1080/11263504.2014.984008
Boratyńska K, Sękiewicz K, Jasińska AK, Tomaszewski D. Effect of geographic range discontinuity on taxonomic differentiation of Abies cilicica. Acta Soc Bot Pol . 2015 ; 84 (4) : 419–430. https://doi.org/10.5586/asbp.2015.037
Bariteau M, Panetsos KP, M’hirit O, Scaltsoyiannes A. Genetic variability of Atlas cedar in comparison with other Mediterranean cedars. Mediterranean Forest. 1999 ; 20(4) : 175-190. https://www.foret-mediterraneenne.org/fr/catalogue/id-712-variabilite-genetique-du-cedre-de-l-atlas-en-comparaison-avec-les-autres-cedres-mediterraneens-
Bou Dagher-Kharrat M, Grenie G, Bariteau M, Brown S, Siljak-Yakolev S, Savouré A. Karyotype analysis reveals inter-specific differentiation in the genus Cedrus despite genome size and base composition constancy. Theor Appl Genet . 2001; 103(6): 846-854. https://doi.org/10.1007/s001220100640
Wodehouse RP. Pollen grains. Their structure, identification and significance in science and medicine. Mac. Graw Hill. New York; 1935. 574 p.
Aytug B. Study of pollens of the cedar genus (Cedrus Link.). Poli. and spores. 1961; 3(1): 47-54.
Erdtman G. An introduction to pollen analysis. Massachusetts: The Chronica Botanica Company. 1943.
Silveira FA. Influence of pollen grain volume on the estimation of the relative importance of its source to bees. Apidologie. 1991; 22: 495-502. https://doi.org/10.1051/apido:19910502
Bercu R, Broasca L, Popoviciu R. Comparative anatomical study of some species leaves. Botanica Serbica. 2010; 34(1): 21–28.
Dörken VM, Stützel T. Morphology, anatomy and vasculature of leaves in Pinus (Pinaceae) and its evolutionary meaning. Flora. 2012; 207: 57-62. https://doi.org/10.1016/j.flora.2011.10.004.
Ghimire B, Lee C, Heo K. Leaf anatomy and its implications for phylogenetic relationships in Taxaceae. J Plant Res. 2014; 127(3): 373-388. https://doi.org/10.1007/s10265-014-0625-3
Woo KS, Fins L, McDonald GI, Wenny DL, Eramian A. Effects of nursery environment on needle morphology of Pinus monticola Dougl. and implications for tree improvement programs. New Forests. 2002; 24:113-129. https://doi.org/10.1023/A:1021230304530
Tiwari SP, Kumar P, Yadav D, Chauhan DK. Comparative morphological, epidermal, and anatomical studies of Pinus roxburghii needles at different altitudes in the North-West Indian Himalayas. Turk J Bot . 2013; (37): 65-73. https://doi.org/10.3906/bot-1110-1
Huang Y, Mao J, Chen Z, Meng J, Xu Y, Duan A, Li Y. Genetic structure of needle morphological and anatomical traits of Pinus yunnanensis. J. For. Res. 2016; 27(1):13-25. https://doi.org/10.1007/s11676-015-0133-x
Begcy K, Dresselhaus T. Epigenetic responses to abiotic stresses during reproductive development in cereals. Plant Reprod. 2018; 31: 343–355.https://doi.org/10.1007/s00497-018-0343-4
Hinojosa L, Matanguihan JB, Murphy KM. Effect of high temperature on pollen morphology, plant growth and seed yield in quinoa (Chenopodium quinoa Willd.). J Agro Crop Sci. 2018; 205: 33-45. https://doi.org/10.1111/jac.12302
Nunney L. The limits to knowledge in conservation genetics-the value of effective population size. Evolutionary Biology. 2000; 32:179-194. https://doi.org/10.1007/978-1-4615-4135-6_9
Nikkanen T, Ruotsalainen S. Variation in flowering abundance and its impact on the genetic diversity of the seed crop in a Norway spruce seed orchard. Silva Fennica. 2000; 34: 205-222. https://doi.org/10.14214/sf.626
Khanduri VP, Sharma CM. Cyclic pollen production in Cedrus deodara. Sex Plant Reprod . 2009; 22(2): 53-61. https://doi.org/10.1007/s00497-008-0091-y
Saouab F-E, Bendriss Amraoui M. Short Shoot Growth and Reproduction Response to Light Conditions Vary with Order Branching in the Proximal Part of C. atlantica Crown. International Journal of Forestry Research. 2020; 2020. https://doi.org/10.1155/2020/8383010
Khanduri VP, Sukumaran A, Sharma CM. Gender plasticity uncovers multiple sexual morphs in natural populations of Cedrus deodara (Roxb.) G. Don. Ecol Process . 2021; 10. https://doi.org/10.1186/s13717-021-00311-7
Fries A. Development of flowering and effect of pruning in a clonal seed orchard of lodgepole pine. Canadian Journal of Forest Research. 1994; 24: 71-76. https://doi.org/10.1139/x94-011
Bila AD, Lindgren D. Fertility variation in Milletia stuhlmannii, Brachystegia spiciformis, Brachystegia bohemii and Leucaena leucocephala and its effects on relatedness in seeds. Forest Genetics. 1998; 5: 119–129.
Smith RW. Life History of Cedrus atlantica. Botanical Gazette. 1923; 75(2): 203-208. https://www.journals.uchicago.edu/doi/10.1086/333157
Alves Rodrigues BR, Nietsche S, Mercadante-Simões MO, Toledo Pereira MC, Ribeiro LM. Climatic seasonality influences the development of pollen grains and fruiting in Annona squamosa. Envion Exp Bot. 2018; 150: 240–248. https://doi.org/10.1016/j.envexpbot.2018.03.025
Cruzan MB. Variation in pollen size, fertilization ability, and postfertilization siring ability in Erythronium grandiflorum. Evolution. 1990; 44(4): 843-856. https://doi.org/10.2307/2409550
Lopez HA, Anton AM, Galetto L. Pollen-pistil size correlation and pollen size-number trade-off in species of Argentinian Nyctaginaceae with different pollen reserves. Plant Syst. Evol. 2005; 256: 69-73. https://doi.org/10.1007/s00606-005-0372-y
Ejsmond MJ, Ejsmond A, Banasiak Ł, Ska-Kołaczek MK, Kozłowski J, Kołaczek P. Large pollen at high temperature: an adaptation to increased competition on the stigma?. Plant Ecol. 2015; 216 :1407-1417. https://doi.org/10.1007/s11258-015-0519-z
Carrizo-García C. Pollen starch reserves in tomato relatives: Ecophysiological implications. Grana. 2007; 46(1): 13-19. https://doi.org/10.1080/00173130601179217
El Bakkali N, Bendriss Amraoui M. Morphological and Anatomical Characterization of Ecotype Needles of Cedrus atlantica in Morocco. Int. J. Fores. Res.. 2022a; 11. https://doi.org/10.1155/2022/5836589
Cobo-Simon I, Gomez-Garrido J, Esteve-Codina A, Dabad M, Alioto T, Maloof JN, Mendez-Cea B, Seco JI, Linares JC, Gallego FJ. De novo transcriptome sequencing and gene co-expression reveal a genomic basis for drought sensitivity and evidence of a rapid local adaptation on Atlas cedar (Cedrus atlantica). Front. Plant Sci. 2023; 14. https://doi.org/10.3389/fpls.2023.1116863
Knight CA, Clancy RB, Gotzenberger L, Dann L, Beaulieu JM. On the relationship between pollen size and genome size. J. Bot. 2010; 2010: 1-7. https://doi.org/10.1155/2010/612017
Frenguelli G, Ferranti F, Tedeschini E, Andreutti R. Volume changes in the pollen grain of Corylus avellana L. (Corylaceae) during development. Grana. 1997; 36: 289-292.https://doi.org/10.1080/00173139709362619
Ejsmond MJ, Pilarek DWS, Ejsmond A, Dragosz-kluska D, Ska-kołaczek MK, Kołaczek K, Kozłowski J. Does climate affect pollen morphology? Optimal size and shape of pollen grains under various desiccation intensity. Ecosphere. 2011; 2(10). https://doi.org/10.1890/ES11-00147.1
Yang CF, Guo YH. Pollen size-number trade-off and pollen-pistil relationships in Pedicularis (Orobanchaceae). Plant Syst. Evol. 2004; 247: 177-185. https://doi.org/10.1007/s00606-004-0165-8
Pinheiro-Costa BK, Mesquita-Neto JN, Rego JO, Schlindwein C. Trade-off between quantity and size of pollen grains in the heterandrous flowers of Senna pendula (Fabaceae). Acta Bot. Bras . 2018; 32(3): 446-453. https://doi.org/10.1590/0102-33062018abb0132
Carrizo-Garcıa C, Guarnieri M, Pacini E. Tomato pollen tube development and carbohydrate fluctuations in the autotrophic phase of growth. Acta Physiol Plant . 2012; 34: 2341-2347. https://doi.org/10.1007/s11738-012-1037-4
Bagcioglu M, Zimmermann B, Kohler A. A multiscale vibrational spectroscopic approach for identification and biochemical characterization of pollen. Plos One. 2015; 10(9). https://doi.org/10.1371/journal.pone.0137899
Stiebing C, Post N, Schindler C, Göhrig B, Lux H, Popp J, Heutelbeck A, Schie IW. Revealing the Chemical Composition of Birch Pollen Grains by Raman Spectroscopic Imaging. Int. J. Mol. Sci . 2022; 23. https://doi.org/10.3390/ijms23095112
Lee SK, Lee J, Jo M, Jeon JS. Exploration of Sugar and Starch Metabolic Pathway Crucial for Pollen Fertility in Rice. Int. J. Mol. Sci . 2022 ; 23 (22). https://doi.org/10.3390/ijms232214091
Kaufmann W. On the variations of starch content in hazel pollen seeds. International Bulletin of the Polish Academy of Sciences and Letters: Class of Mathematical and Natural Sciences: Series B: Natural Sciences. 1920: 191-198.
Pacini E, Dolferus R. Pollen Developmental Arrest: Maintaining Pollen Fertility in a World With a Changing Climate. Front. Plant Sci. 2019; 10. https://doi.org/10.3389/fpls.2019.00679
Wilson ZA, Song J, Taylor B, Yang C. The final split: the regulation of anther dehiscence. J. Exp. Bot.. 2011; 62: 1633-1649. https://doi.org/10.1093/jxb/err014
Nelson MR, Band LR, Dyson RJ, Lessinnes T, Wells DM, Yang C, Everitt NM, Jensen OE, Wilson ZA. A biomechanical model of anther opening reveals the roles of dehydration and secondary thickening. New Phytol. 2012; 96: 1030-1037. https://doi.org/10.1111/j.1469-8137.2012.04329.x
Franchi GG, Bellani L, Nepi M, Pacini E. Types of carbohydrate reserves in pollen: Localization, systematic distribution and ecophysiological significance. Flora. 1996; 191(2): 143–159. https://doi.org/10.1016/S0367-2530(17)30706-5
Buchmann SL. Vibratile pollination in Solanum and Lycopersicon: A look at pollen chemistry. In: D’Arcy WG (Eds.). Solanaceae II: Biology and systematics. New York: Columbia Univ. Press; 1986. 237-252 p. https://digitalcommons.usu.edu/bee_lab_bo/255
Firon N, Nepi M, Pacini E. Water status and associated processes mark critical stages in pollen development and functioning. Annals of Botany. 2012; 109: 1201-1214. https://doi.org/10.1093/aob/mcs070
Claeys H, Inze D. The agony of choice: how plants balance growth and survival under water-limiting conditions. Plant Physiol . 2013; 162: 1768–1779. https://doi.org/10.1104/pp.113.220921
Hoekstra F, Golovina E, Tetteroo F, Wolkers W. Induction of desiccation tolerance in plant somatic embryos: how exclusive is the protective role of sugars?. Cryobiology. 2001; 43: 140-150. https://doi.org/10.1006/cryo.2001.2358
Anil Kumar S, Hima Kumari P, Shravan Kumar G, Mohanalatha C, Kavi Kishor PB. Osmotin: a plant sentinel and a possible agonist of mammalian adiponectin. Front. Plant Sci. 2015; 6. https://doi.org/10.3389/fpls.2015.00163
Pérez Di Giorgio JA. Pollen aquaporins: What are they there for?. Plant Signalling & Behavior. 2016; 11. https://doi.org/10.1080/15592324.2016.1217375
Dimou M, Liolios V, Rodopoulou MA, Tananaki C. The effect of pollen grain morphology on sugars content of bee-collected pollen: the
significance of size and exine ornamentation. Grana. 2020; 59(5): 389-395. https://doi.org/10.1080/00173134.2020.1734651
Vesprini JL, Nepi M, Cresti L, Guarnieri M, Pacini E. Changes in cytoplasmic carbohydrate content during Helleborus pollen presentation. Grana. 2002; 41(1): 16-20. https://doi.org/10.1080/00173130260045459
Baker HG, Baker I. Starch in angiosperm pollen grains and its evolutionary significance. Am J Bot. 1979; 66: 591–600. https://doi.org/10.1080/0028825X.1979.10432569
Cruden RW. Pollen grain size, stigma depth, and style length: The relationships revisited. Plant Syst Evol. 2009; 278(3) :223-238. https://doi.org/10.1007/s00606-008-0142-8
Rounds CM, Lubeck E, Hepler PK, Winship LJ. Opidium iodide competes with Ca (2+) to label pectin in pollen tubes and Arabidopsis root hairs. Plant Physiology. 2011; 157(1):175-187. https://doi.org/10.1104/pp.111.182196
Nygaard P. Studies on the Germination of Pine Pollen (Pinus mugo) in vitro. I. Growth Conditions and Effects of pH and Temperature on Germination, Tube Growth and Respiration. Physiologia Plantarum. 1969; 22: 338-346. https://doi.org/10.1111/j.1399-3054.1969.tb07384.x
Krichevsky A, Kozlovsky SV, Tian GW, Chen MH, Zaltsman A, Citovsky V. How pollen tubes grow. Developmental Biology. 2006; 303(2): 405-420. https://doi.org/10.1016/j.ydbio.2006.12.003
Fernando D, Owens JN, Yu X, Ekramoddoullah AKM. RNA and protein synthesis during in vitro pollen germination and tube elongation in Pinus monticola and other conifers. Sex Plant Reprod. 2001; 13(5):259-264. https://doi.org/10.1007/s004970100069
Niklas KJ. Plant biomechanics: an engineering approach to plant form and function. University of Chicago Press, Chicago, Illinois, USA, 1992.
Schwendemann AB, Wang G, Mertz ML, Mcwilliams RT, Thatcher SL, Osborn JM. Aerodynamics of saccate pollen and its implications for wind pollination. Am. J. Bot. 2007; 94(8): 1371–1381. https://doi.org/10.3732/ajb.94.8.1371
Salter J, Murray BG, Raggins JE. Wettable and unsinkable: the hydrodynamics of saccate pollen grains in relation to the pollination mechanism in the two New Zealand species of Prumnopitys Phil. (Podocarpaceae). Annals of Botany. 2002; 89: 133-144.
Leslie AB. Flotation preferentially selects saccate pollen during conifer pollination. New Phytologist. 2010; 188: 273-279.
https://doi.org/10.1111/j.1469-8137.2010.03356.x
Lu Y, Jin B, Wang L, Wang Y, Wang D, Jiang XX, Chen P. Adaptation of male reproductive structures to wind pollination in gymnosperms: Cones and pollen grains. Canadian J Plant Sci. 2011; 91(5): 897-906. https://doi.org/10.1139/CJPS2011-020