Evaluation of Schiff Bases Against Two Cancer Cell Lines: A Combined Experimental and Theoretical Approach
Main Article Content
Abstract
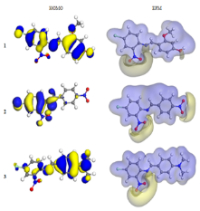
The rising global cancer rate necessitates the development of new therapeutic agents, with Schiff bases, compounds containing primary amines and aldehydes, gaining attention for their anticancer properties. The present study investigated the anticancer potential of three Schiff base derivatives against two cancer cell lines, MDA-MB231 (breast cancer) and HepG2 (liver cancer), employing both experimental and theoretical approaches. Schiff bases were synthesized using condensation reactions and characterized employing 1H-NMR, 13C-NMR, and mass spectrometry. Cytotoxicity was evaluated using the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, and determined IC50 values. Computational studies using density functional theory (DFT) were employed to optimize molecular structures and assess electronic properties of Schiff bases, computing HOMO-LUMO energy gaps, dipole moments, and heat of formation. The three Schiff bases synthesized included (E)-4-(((4-chloro-2-nitrophenyl)imino)methyl)-3-methoxyphenol (1), 2-((2,4-dichlorophenylimino)methyl)-5-(diethylamino)phenol (2), and (E)-4-(((4-chloro-2-nitrophenyl)imino)methyl)-3-methoxy-N,N-dimethylaniline (3). Compound 2 demonstrated the highest activity against the HepG2 cell line with an IC50 value of 43.17 µg/mL, while compounds 1 and 3 exhibited lower activities (IC50 values of 70.29 and 73.69 µg/mL, respectively). The compounds also showed varying degrees of activity against the MDA-MB231 cell line, with compound 2 being the most effective. Molecular docking simulations further supported the experimental results, highlighting significant binding interactions between compound 2 and breast cancer-related proteins, with the strongest binding observed to Akt (5KCV). Similarly, compound 1 demonstrated effective binding to liver cancer proteins. This combined experimental and theoretical study provides valuable insights into the anticancer activity of Schiff bases and highlights their potential as promising candidates for cancer therapy.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Mohammed KK, Almasha FA, Al-hujaj HH, Al-asadi RH, Jaber HA, Jassem AM, Dhaef HK. Synthesis, characterization, cytotoxic evaluation on MCF-7 breast cancer cells, and theoretical studies of novel 1,2,3-triazoles. Trop J Nat Prod Res. 2024;8(10):8788-8795.
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4(1):1-18.
Majeda AA, Dunya A, Omran HA, Abbas S, Abide DS, Hmoodf AY. Synthesis, molecular docking of new amide thiazolidine derived from isoniazid and studying their biological activity against cancer cells. J Biomol Struct Dyn. 2023.
Fang X, Cao J, Shen A. Advances in anti-breast cancer drugs and the application of nano-drug delivery systems in breast cancer therapy. J Drug Deliv Sci Technol. 2020;57:101662.
Malik JA, Ahmed S, Jan B, Bender O, Al Hagbani T, Alqarni A, Anwar S. Drugs repurposed: An advanced step towards the treatment of breast cancer and associated challenges. Biomed Pharmacother. 2022;145:112375.
Majed AA, AL-Duhaidahawi D, Omran HA, Abbas S, Abid DS, Hmood AY. Synthesis, molecular docking of new amide thiazolidine derived from isoniazid and studying their biological activity against cancer cells. J Biomol Struct Dyn. Published online 2023.
Meena SK, Sanjeet S. A comparative thermal stability study of N-(4-nitropheny) maleimide and N-(2,4-di-nitrophenyl) maleimide with methylacrylate. Int J Res Appl Sci Eng Technol. 2023;11(1):1574-1585.
Muhammad P, Musarat S, Anam E, Rimsha Q, Zohaib S, Adnan A, Khan RR, Bukhari SM, Sami U, Umer Y. An overview of aniline-based Schiff base metal complexes: synthesis, characterization, and biological activities—a review. Inorg Chem Commun. 2023;159:111851.
Abdul H, Mariya A, Maliha U, Ali SA, Khan KM. Schiff bases in medicinal chemistry: a patent review (2010-2015). Expert Opin Ther Pat. 2017;27(1):63-79.
Singh N, Rastogi S, Singh RV. Microwave-assisted synthesis and antibacterial and pesticidal activity studies of aluminum (III) and gallium (III) complexes with a bioactive Schiff base. Main Group Met Chem. 2011;34(5-6):147-152.
Jai D, Jyoti Y, Kashmiri L, Nikhil K, Paul AK, Deepak K, Dutta PP, Jindal DK. Design, synthesis, crystal structure, molecular docking studies of some diorganotin(IV) complexes derived from the piperonylic hydrazide Schiff base ligands as cytotoxic agents. J Mol Struct. 2021;1232(15):129992.
Magalhães TFF, da Silva CM, dos Santos LBF, Silva LM, Fuchs BB, Mylonakis E, Martins CVB, de Resende-Stoianoff MA, de Fátima Â. Cinnamyl Schiff bases: synthesis, cytotoxic effects, and antifungal activity of clinical interest. Lett Appl Microbiol. 2020;71(5):490-497.
Odularu AT. Manganese Schiff base complexes, crystallographic studies, anticancer activities, and molecular docking. J Chem. 2022.
Jain S, Rana M, Sultana R, Mehandi R, Rahisuddin. Schiff base metal complexes as antimicrobial and anticancer agents. Polycycl Aromat Compd. 2023;43(7):6351-6406.
Domenico I, Jessica C, Alessia C, Annaluisa M, Federica G, Carmela S, Pasquale L, Maria SS. Metal complexes with Schiff bases as antimicrobials and catalysts. Inorganics (Basel). 2023;11(8).
You ZL, Cui YM, Ma YP, Wang C, Zhou XS, Li K. Synthesis, characterization, and urease inhibitory activity of oxovanadium(V) complexes with similar Schiff bases. Inorg Chem Commun. 2011;14(5):636-640.
Rashid A, Momin K, Aftab A, Elhenawy AA, AlAsmari AF, Metab A, Fawaz A, Manzoor A. Synthesis, molecular structure, and urease inhibitory activity of novel bis-Schiff bases of benzyl phenyl ketone: a combined theoretical and experimental approach. Saudi Pharm J. 2023;31(8):101688.
Krishna GA, Dhanya TM, Shanty AA, Raghu KG, Mohanan PV. Transition metal complexes of imidazole derived Schiff bases: antioxidant/anti-inflammatory/antimicrobial/enzyme inhibition and cytotoxicity properties. J Mol Struct. 2023;1274.
Qurat-Ul-Ain S, Muhammad P, Abdul M, Umer Y, Zohaib S, Adnan A, Khan RR, Sami U, Faisal A, Seemal J. Review: Schiff base metal complexes as anti-inflammatory agents. J Coord Chem. 2023;76(9-10):1094-1118.
Alshamrani M. Medicinal importance and chemosensing applications of Schiff base derivatives for the detection of metal ions: a review. Main Group Chem. 2023;22(2):251-280.
Al-hujaj HH, Almashal FA, Kadum AT, Mohammed KK, Hussein KA, Jassem AM. Click chemistry-based synthesis of novel 1,2,3-triazole derivatives and cytotoxic activity on breast and prostate cancer cell lines. Trop J Nat Prod Res. 2023;7:3306-3313.
Aytac S, Gundogdu O, Bingol Z, Gulcin İ. Synthesis of Schiff bases containing phenol rings and investigation of their antioxidant capacity, anticholinesterase, butyrylcholinesterase, and carbonic anhydrase inhibition properties. Pharmaceutics. 2023;15(3):779.
Shah SS, Shah D, Khan I, Ahmad S, Ali U, Rahman AU. Synthesis and antioxidant activities of Schiff bases and their complexes: an updated review. Biointerface Res Appl Chem. 2020;10(6):6936-6963.
Aulakh JS, Kumar V, Kim KH. A review of the applications of Schiff bases as optical chemical sensors. Trends Anal Chem. 2019;116:74-91.
Berhanu AL, Mohiuddin I, Malik AK, Aulakh JS, Kumar V, Kim KH. A review of the applications of Schiff bases as optical chemical sensors. TrAC Trends Anal Chem. 2019;116:74-91.
Khan Z, Tanoli MAK. Exploring the legacy: the biological interactions of bis-Schiff bases and their coordinated azomethine derivatives over time. Pak J Med Dent. 2024;13(2):90-101.
Yeğiner G, Gülcan M, Işık S, Ürüt GÖ, Özdemir S, Kurtoğlu M. Transition metal (II) complexes with a novel azo-azomethine Schiff base ligand: synthesis, structural and spectroscopic characterization, thermal properties, and biological applications. J Fluoresc. 2017; 27:2239-2251.
Almashal FA, Al-Abdullah ZT, Jassem AM. Schiff base synthesis as a capping agent for green synthesized silver nanoparticles. Egypt J Chem. 2020;63(3):813-821.
Reyhaneh F, Maryam S, Jayakumar R, Abdulla MA, Hashim NB, Khaing SL, Salehen NB. In vivo acute toxicity evaluation and in vitro molecular mechanism study of antiproliferative activity of a novel indole Schiff base β-diiminato manganese(III) complex in hormone-dependent and triple-negative breast cancer cells. PeerJ. 2019;(10).
Al-Asadi RH, Mohammed KK, Dhaef HK. Mercuration and telluration of 2-fluoro-5-nitroaniline: synthesis, antibacterial, and computational study. Russ J Gen Chem. 2020;90(4):703-709.
Al-Asadi RH. Synthesis, DFT calculation, and biological activity of some organotellurium compounds containing azomethine group. Orbital. 2019;11(7):402-410.
Al-Ameertaha RA, Al-Asadi RH. Design, synthesis, characterization, DFT, molecular docking studies, and evaluation of biological activity of benzamidethiourea derivatives against HepG2 hepatocellular carcinoma cell lines. Adv J Chem Sect A. 2025;8(2):220-244.
Mutlaq DZ, Al-Shawi AAA, Al-Asadi RH. Synthesis, characterization, anticancer activity, and molecular docking of novel maleimide-succinimide derivatives. Egypt Pharm J. 2021;20(4):303-312.
Nasser AT, Al-Asadi RH. Schiff base ligands derived from o-phthalaldehyde and their metal complexes with Cu2+ and Ni2+: synthesis, anti-breast cancer and molecular docking study. Trends Sci. 2023;20(9):5675-5685.
Al-Salim YM, Al-Asadi RH. Synthesis, anti-breast cancer activity, and molecular docking studies of thiourea benzamide derivatives and their complexes with copper ion. Trop J Nat Prod Res. 2023;7(6):3158-3167.
Al-Ameertaha RA, Al-Asadi RH. Synthesis, characterization, and anticancer evaluation of thiourea benzamide derivatives and their Cu (II) and Pt (IV) complexes against PC3 and HepG2 cancer cell lines. Trop J Nat Prod Res. 2024;8(11):9050-9060.