In Silico and Multivariate Analysis of Herbal Compounds in Asthma Inflammation: Exploring Alternatives to Corticosteroids
Main Article Content
Abstract
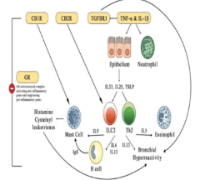
Corticosteroids are among the most common therapies for asthma-controlled treatment but, due to their side effects, alternative therapies are being developed. This in silico study intends to identify the distinct anti-asthma inflammatory activity of corticosteroids (budesonide and prednisolone) to herbal compounds (cannabidiol, andrographolide, and eucalyptol) toward GR, CB1R, CB2R, TNF-α, IL-1β, and TGFBR1 targets. In silico studies were carried out using PLANTS and AutoDock software. Additional software such as YASARA, MarvinSketch, BIOVIA Discovery Studio Visualizer, and PyMol were used for docking preparation and visualization. RStudio was used to perform multivariate analysis of binding affinity values. ADME characteristics were predicted using pkCSM. Both docking applications identified the TNF-α-eucalyptol interaction as the weakest binding affinity and the CB2-reference exhibited the strongest. The highest binding scores in PLANTS and AutoDock were -133.38 and -13.75 kcal/mol, respectively, while the lowest were -48.12 and -4.19 kcal/mol. Budesonide and prednisolone's binding activities were closest to cannabidiol and andrographolide (similarity>73%). In comparison to other chemicals, eucalyptol has demonstrated the most distinct affinity to the targets (similarity<50%). From all the ligands’ ADME characteristics, prednisolone potentially offers the most comprehensive benefits in asthma inflammation treatment, although with higher risk of side effects than budesonide. On the other hand, the herbal compounds demonstrate profiles suitable for systemic therapy, with differences in distribution and clearance that influence their action and side effects. In conclusion, the herbal compounds could be alternative therapies to budesonide and prednisolone in asthma. However, eucalyptol predictably has lower activity.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Wang Z, Li Y, Gao Y, Fu Y, Lin J, Lei X, Zheng J, Jiang M. Global, regional, and national burden of asthma and its attributable risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Respir Res. 2023; 24(1):1–13.
Dharmage SC, Perret JL, Custovic A. Epidemiology of Asthma in Children and Adults. Front Pediatr [Internet]. 2019; 7:1–15.
Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015; 16(1):45–56.
Birmann PT, Casaril AM, Zugno GP, Acosta GG, Severo Sabedra Sousa F, Collares T, Seixas FK, Jacob RG, Brüning CA, Savegnago L, Hartwig D. Flower essential oil of Tagetes minuta mitigates oxidative stress and restores BDNF-Akt/ERK2 signaling attenuating inflammation- and stress-induced depressive-like behavior in mice. Brain Res. 2022;1784:1-12.
Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNFα in pulmonary pathophysiology. Respir Res. 2006; 7(1):125–133.
Kraik K, Tota M, Laska J, Łacwik J, Paździerz Ł, Sędek Ł, Gomułka K. The role of transforming growth factor-β (TGF-β) in asthma and chronic obstructive pulmonary disease (COPD). 2024; 13(15):1271–1293.
Palomares O. Could we co-opt the cannabinoid system for asthma therapy? Expert Rev Clin Immunol. 2023; 19(10):1183–1186.
Vuolo F, Abreu SC, Michels M, Xisto DG, Blanco NG, Hallak JE, Zuardi AW, Crippa JA, Reis C, Bahl M, Pizzichinni E, Maurici R, Pizzichinni MMM, Rocco PRM, Dal-Pizzol F. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur J Pharmacol. 2019; 843:251–259.
Global Initiative for Asthma. Global strategy for asthma management and prevention. 2024. Available from: https://ginasthma.org/2024-report/
Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008; 8(3):218–30.
Williams DM. Clinical pharmacology of corticosteroids. Respir Care. 2018; 63(6):655–670.
Patel SS, Savjani JK. Systematic review of plant steroids as potential antiinflammatory agents: Current status and future perspectives. J Phytopharm. 2015; 4(2):121–125.
Bao Z, Guan S, Cheng C, Wu S, Wong SH, Kemeny DM, Leung, Bernard P, Wong WSF. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-κB pathway. Am J Respir Crit Care Med. 2009; 179(8):657–665.
Juergens LJ, Worth H, Juergens UR. New perspectives for mucolytic, anti-inflammatory and adjunctive therapy with 1,8-Cineole in COPD and asthma: Review on the new therapeutic approach. Adv Ther. 2020; 37(5):1737–1753.
Galgale S, Zainab R, Kumar A. P, M. N, D. S, Sharma S. Molecular docking and dynamic simulation-based screening identifies inhibitors of targeted SARS-CoV-2 3CLpro and human ACE2. Int J Appl Pharm. 2023; 297–308.
Granato D, Santos JS, Escher GB, Ferreira BL, Maggio RM. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci Technol. 2018; 72:83–90.
Nugroho AA, Hadi MS, Adianto C, Putra JAK, Purnomo H, Fakhrudin N. Molecular docking and ADMET prediction studies of flavonoids as multi-target agents in COVID-19 therapy: anti-inflammatory and antiviral approaches. Indones J Pharm. 2023; 34(4):651–664.
Tjitda PJP, Nitbani FO, Parikesit AA, Bessi MIT, Wahyuningsih TD. In silico investigation of tropical natural product for wild-type and quadrupole mutant PfDHFR inhibitors as antimalarial candidates. Trop J Nat Prod Res. 2024; 8(2):6208–6217.
Mulyati B, Panjaitan RS. Study of molecular docking of alkaloid derivative compounds from stem karamunting (Rhodomyrtus tomentosa) against α-glucosidase enzymes. Indones J Chem Res. 2021;9(2):129–136.
Asnawi A, Nedja M, Febrina E, Purwaniati P. Prediction of a stable complex of compounds in the ethanol extract of celery leaves (Apium graveolens L.) function as a VKORC1 antagonist. Trop J Nat Prod Res. 2023; 7(2):2362–2370.
Nur S, Hanafi M, Setiawan H, Nursamsiar N, Elya B. Molecular docking simulation of reported phytochemical compounds from Curculigo latifolia extract on target proteins related to skin antiaging. Trop J Nat Prod Res. 2023; 7(11):5067–5080.
Korb O, Stützle T, Exner TE. An ant colony optimization approach to flexible protein–ligand docking. Swarm Intell. 2007; 1(2):115–134.
Korb O, Stützle T, Exner TE. Empirical scoring functions for advanced protein−ligand docking with PLANTS. J Chem Inf Model. 2009; 49(1):84–96.
Hill AD, Reilly PJ. Scoring functions for autoDock. In: Lütteke T, Frank M, editors. Glycoinformatics. New York, NY: Springer New York; 2015. p. 467–474. (Methods in Molecular Biology; vol. 1273).
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009; 30(16):2785–2791.
Pires DEV, Blundell TL, Ascher DB. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem. 2015; 58(9):4066–4072.
Su M, Yang Q, Du Y, Feng G, Liu Z, Li Y, Wang R. Comparative assessment of scoring functions: The CASF-2016 Update. J Chem Inf Model. 2019; 59(2):895–913.
Zheng L, Meng J, Jiang K, Lan H, Wang Z, Lin M, Li W, Guo H, Wei Y, Mu Y. Improving protein–ligand docking and screening accuracies by incorporating a scoring function correction term. Brief Bioinform. 2022; 23(3):1–15.
Agistia DD, Purnomo H, Tegar M, Nugroho AE. Interaction between active compounds from Aegle marmelos Correa as anti-inflammation agent with COX-1 and COX-2 receptor. Tradit Med J. 2013; 18(2):80–87.
Ivanova L, Karelson M. The impact of software used and the type of target protein on molecular docking accuracy. Molecules. 2022; 27(24):9041–9060.
Korb O, Stützle T, Exner TE. PLANTS: Application of ant colony optimization to structure-based drug design. In: Dorigo M, Gambardella LM, Birattari M, Martinoli A, Poli R, Stützle T, editors. Ant colony optimization and swarm intelligence. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006. p. 247–258.
David CC, Jacobs DJ. Principal Component Analysis: A method for determining the essential dynamics of proteins. In: Livesay DR, editor. Protein Dynamics. Totowa, NJ: Humana Press; 2014. p. 193–226.
Ryu J, Liu K biu, McCloskey TA. The use of multivariate PCA dataset in identifying the underlying drivers of critical stressors, looking at global problems through a local lens. Data Brief. 2022;41(2022):1–12.
Febrina E, Alamhari RK, Abdulah R, Lestari K, Levita J, Supratman U. Molecular docking and molecular dynamics studies of Acalypha indica L. phytochemical constituents with caspase-3. Int J Appl Pharm. 2021;210–215.
Castel B, Tomlinson L, Locci F, Yang Y, Jones JDG. Optimization of T-DNA architecture for Cas9-mediated mutagenesis in Arabidopsis. Lai EM, editor. PLOS ONE. 2019;14(1):1–20.
Lisdiana L, Widiatningrum T, Kurniawati F. Original research article molecular docking bioactive compound of rambutan peel (Nephelium lappaceum L) and NF-Κb in the context of cigarette smoke-induced inflammation. 2022;6(10):1654–1659.
Inayati I, Arifin NH, Febriansah R, Indarto D, Suryawati B, Hartono H. Trans-Cinnamaldehyde inhibitory activity against mrka, trec, and luxs genes in biofilm-forming Klebsiella pneumoniae: An in silico study. Trop J Nat Prod Res. 2023; 7(10):4249–4255.
Putra OA, Rahmania TA, Renesteen E. Molecular docking study as therapeutic approach for targeting cholecystokinin in pancreatic cancer. Int J Appl Pharm. 2024; 340–349.
Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9 ‐tetrahydrocannabinol, cannabidiol and Δ9 ‐tetrahydrocannabivarin. Br J Pharmacol. 2008; 153(2):199–215.
Hidalgo MA, Hancke JL, Bertoglio JC, Burgos RA. Andrographolide a new potential drug for the long term treatment of rheumatoid arthritis disease. In: Matsuno H, editor. Innovative rheumatology. InTech; 2013.
Bhowal M, Gopal M. Eucalyptol: Safety and pharmacological profile. RGUHS J Pharm Sci. 2016; 5(4):125–131.
Derendorf H, Nave R, Drollmann A, Cerasoli F, Wurst W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J. 2006; 28(5):1042–1050.
Derendorf H, Hochhaus G, Meibohm B, Möllmann H, Barth J. Pharmacokinetics and pharmacodynamics of inhaled corticosteroids. J Allergy Clin Immunol. 1998 ; 101(4):440–446.
Kelly HW. Potential adverse effects of the inhaled corticosteroids. J Allergy Clin Immunol. 2003; 112(3):469–478.
Klimoszek D, Jeleń M, Dołowy M, Morak-Młodawska B. Study of the lipophilicity and ADMET parameters of new anticancer diquinothiazines with pharmacophore substituents. Pharmaceuticals. 2024; 17(6):725–745.
Rahardhian MRR, Susilawati Y, Musfiroh I, Febriyanti RM, Muchtaridi, Sumiwi SA. In silico study of bioactive compounds from sungkai (Peronema canescens) as immunomodulator. Int J Appl Pharm. 2022; 135–141.
Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991; 175(3):880–885.
Vandamme. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int J Nanomedicine. 2008; 3(1):1–19.
Petereit AC, Swinney K, Mensch J, Mackie C, Stokbroekx S, Brewster M, Dressman JB. Prediction of blood–brain barrier penetration of poorly soluble drug candidates using surface activity profiling. Eur J Pharm Biopharm. 2010; 75(3):405–410.
Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008; 9(4):310–322.
Korzekwa K, Nagar S. Process and system clearances in pharmacokinetic models: Our basic clearance concepts are correct. Drug Metab Dispos. 2023; 51(4):532–542.
Hsu CH, Hsu CL, Langley A, Wojcik C, Iraganje E, Grygiel-Górniak B. Glucocorticoid-induced osteoporosis—from molecular mechanism to clinical practice. Drugs Ther Perspect. 2024; 40(8):315–329.
Meier CA. Mechanisms of immunosuppression by glucocorticoids. Eur J Endocrinol. 1996; 134(1):50.
Pofi R, Caratti G, Ray DW, Tomlinson JW. Treating the side effects of exogenous glucocorticoids; Can we separate the good from the bad ? Endocr Rev. 2023; 44(6):975–1011.
O’Sullivan SE, Yates AS, Porter RK. The peripheral cannabinoid receptor type 1 (CB1) as a molecular target for modulating body weight in man. Molecules. 2021; 26(20):6178–6192.
Wang J, Lu H xia, Wang J. Cannabinoid receptors in osteoporosis and osteoporotic pain: a narrative update of review. J Pharm Pharmacol. 2019; 71(10):1469–1474.
Basu S, Dittel BN. Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol Res. 2011; 51(1):26–38.
Evangelatos G, Bamias G, Kitas GD, Kollias G, Sfikakis PP. The second decade of anti-TNF-a therapy in clinical practice: new lessons and future directions in the COVID-19 era. Rheumatol Int. 2022; 42(9):1493–1511.
Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, Nanes MS. Inhibition of osteoblast differentiation by tumor necrosis factor-α*. Endocrinology. 2000; 141(11):3956–3964.
Patsalos O, Dalton B, Leppanen J, Ibrahim MAA, Himmerich H. Impact of TNF-α inhibitors on body weight and BMI: A systematic review and meta-analysis. Front Pharmacol. 2020; 11(481):1–15.
Osei ET, Brandsma CA, Timens W, Heijink IH, Hackett TL. Current perspectives on the role of interleukin-1 signalling in the pathogenesis of asthma and COPD. Eur Respir J. 2020; 55(2):1–16.
De Mooij CEM, Netea MG, Van Der Velden WJFM, Blijlevens NMA. Targeting the interleukin-1 pathway in patients with hematological disorders. Blood. 2017; 129(24):3155–3164.
Tseng HW, Samuel SG, Schroder K, Lévesque JP, Alexander KA. Inflammasomes and the IL-1 family in bone homeostasis and disease. Curr Osteoporos Rep. 2022; 20(3):170–185.
Wu M, Chen G, Li YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016; 4(1):1–21.
Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-β pathway. J Hematol OncolJ Hematol Oncol. 2021; 14(1):1-20.