Potential Antiplasmodial Activity of Artemether and Miconazole Combination against Plasmodium berghei in Preclinical Murine Malaria Model Tropical Journal of Natural Product Research
Main Article Content
Abstract
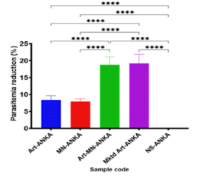
Following recent reports of resistance to currently approved artemisinin-based combination therapy (ACT), there is an urgent need for alternative ACT for effective treatment of malaria. Meanwhile, the antimalarial potential of artemether/miconazole combination has not been explored. In this work, we investigated artemether/miconazole combination as a new combo-therapy for malaria caused by chloroquine-sensitive and multidrug-resistant Plasmodium berghei (Pb) in murine models. The antimalarial activity of each drug and their combination [artemether (8mg/kg)/miconazole (2mg/kg)] was investigated using standard protocols for uncomplicated malaria (UM) and severe malaria (SM) in mice infected with chloroquine-sensitive Pb (CPb) and Pb ANKA (PbA), respectively. Hematological parameters (WBC, RBC, PCV and haemoglobin) and lethality of infected mice were assessed. Results revealed that the combination administered peorally (p.o.) gave greater antimalarial activity (p<0.0001) than monotherapies of pure artemether, miconazole and marketed chloroquine (p.o) although the effect was less than that of therapeutic dosage of marketed ACT (artemether-lumefantrine) (4mg/24mg/kg) against CPb. Furthermore, intraperitoneally (i.p.) administered artemether/miconazole combo-therapy gave greater antimalarial activity than artemether and miconazole monotherapies (p<0.0001) and the effect was comparable with commercial i.m. artemether against PbA. Moreover, the combo-therapies had similar effects to conventional antimalarials in preventing Pb-induced alterations in hematological parameters of the malariogenic mice. Results indicate miconazole as a promising repurposable drug with therapeutic potential against drug-sensitive and resistant parasites. Therefore, artemether/miconazole combination could serve as a plausible ACT option for UM and as an alternative to artemisinin derivatives in SM. An on-going research would seek to enhance the observed effects via nanotechnology-based drug delivery systems.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Fried M, Duffy PE. Malaria during pregnancy. Cold Spring Harb. Perspect. Med. 2017;7(6):1–24.
Dayananda KK, Achur RN, Gowda DC. Epidemiology, drug resistance, and pathophysiology of Plasmodium vivax malaria. J. Vector Borne Dis. 2018;55(1):1–8.
World Health Organization. World Malaria Report: 20 years of global progress and challenges. 2020. Available online: https://www.who.int/publications/i/item/9789240015791
Al-Awadhi M, Ahmad S, Iqbal J. Current status and the epidemiology of malaria in the Middle East region and beyond. Microorg. Rev. 2021;9(338):1–20.
Chikezie P. Critical roles of thiol-mediated antioxidant detoxification systems in the pathophysiology of Plasmodium falciparum-infected erythrocytes. J. Investig. Biochem. 2015;4(3): 61. doi: 10.5455/jib.20151230060340.
Wassmer SC, Taylor TE, Rathod PK, Mishra SK, Mohanty S, Arevalo-Herrera M, Duraisingh MT, Smith JD. Investigating the pathogenesis of severe malaria: A multidisciplinary and cross-geographical approach. Am. J. Trop. Med. Hyg. 2015;93(3):42–56. doi: 10.4269/ajtmh.14-0841.
Varo R, Crowlev VM, Sitoe A, Madrid L, Serghides L, Kain KC, Bassat Q. Adjunctive therapy for severe malaria: A review and critical appraisal. Malar. J. 2018;17(17):1–18. doi: 10.1186/s12936-018-2195-7.
Mackintosh CL, Beeson JG, Marsh K. Clinical features and pathogenesis of severe malaria. Trends Parasitol. 2004;20(12):597–603. doi: 10.1016/j.pt.2004.09.006.
Balaji SN, Deshmukh R, Trivedi V. Severe malaria: Biology, clinical manifestation, pathogenesis and consequences. J. Vector Borne Dis. 2020;57:1–13.
Maiga FO, Wele M, Toure SM, Keita M, Tangara CO, Refeld RR, Thiero O, Kayentao K, Diakite M, Dara A, Li J, Toure M, Sagara I, Djimde A, Mather FJ, Doumbia SO, Shaffer JG. Artemisinin-based combination therapy for uncomplicated Plasmodium falciparum malaria in Mali: a systematic review and meta-analysis. Malar. J. 2021;20(1):1–13. doi: 10.1186/s12936-021-03890-0.
World Health Organization. Artemisinin resistance and artemisinin-based combination therapy efficacy. In World Health Organization Report. 2019. Available online: https://www.who.int/malaria/publications/atoz/artemisinin-resistance-august2018/en/
Peto TJ, Tripura R, Callery JJ, Lek D, Dang H, Nghia T, Nguon C, Thi N, Thuong H. Triple therapy with artemether – lumefantrine plus amodiaquine versus artemether – lumefantrine alone for artemisinin-resistant, uncomplicated falciparum malaria: an open-label , randomized, multicentre trial. The Lancet Infect. Dis. 2022;22(6):867–878. https://doi.org/10.1016/S1473-3099(21)00692-7
Yavo W, Faye B, Kuete T, Djohan V, Oga SA, Kassi RR, Diatta M, Ama MV, Tine R, Ndiaye JL, Evi JB, Same-Ekobo A, Faye O, Koné M. Multicentric assessment of the efficacy and tolerability of dihydroartemisinin-piperaquine compared to artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in sub-Saharan Africa. Malar. J. 2011;10(198):1–8. https://doi.org/10.1186/1475-2875-10-198
Zwang J, Olliaro P, Barennes H, Bonnet M, Brasseur P, Bukirwa H, Cohuet S, D’Alessandro U, Djimdé A, Karema C, Guthmann JP, Hamour S, Ndiaye JL, Mårtensson A, Rwagacondo C, Sagara I, Same-Ekobo A, Sirima SB, Van Den Broek I, Randrianarivelojosia M. Efficacy of artesunate-amodiaquine for treating uncomplicated falciparum malaria in sub-Saharan Africa: A multi-centre analysis. Malar. J. 2009; 8(1):1–18.
https://doi.org/10.1186/1475-2875-8-203
Dentinger CM, Rakotomanga TA, Rakotondrandriana A, Rakotoarisoa A, Rason MA, Moriarty LF, Steinhardt LC, Kapesa L, Razafindrakoto J, Svigel SS, Lucchi NW, Udhayakumar V, Halsey ES, Ratsimbasoa CA. Efficacy of artesunate-amodiaquine and artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria in Madagascar, 2018. Malar. J. 2021; 20(1), 1–11. https://doi.org/10.1186/s12936-021-03935-4
Duru V, Khim N, Leang R, Kim S, Domergue A, Kloeung N, Ke S, Chy S, Eam R, Khean C, Loch K, Ken M, Lek D, Beghain J, Ariey F, Guerin PJ, Huy R, Mercereau-Puijalon O, Witkowski B, Menard D. Plasmodium falciparum dihydroartemisinin-piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: Retrospective and prospective investigations. BMC Med. 2015; 13(1), 1–11. https://doi.org/10.1186/s12916-015-0539-5
Ye R, Zhang Y, Zhang D. Evaluations of candidate markers of dihydroartemisinin-piperaquine resistance in Plasmodium falciparum isolates from the China–Myanmar, Thailand–Myanmar, and Thailand–Cambodia borders. Parasit Vectors. 2022;15(1):1–9. doi: 10.1186/s13071-022-05239-1.
Marwa K, Kapesa A, Baraka V, Konje E, Kidenya B. Therapeutic efficacy of artemether-lumefantrine, artesunate-amodiaquine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Sub-Saharan Africa: A systematic review and meta-analysis. PLoS One. 2022; 17(3): e0264339. https://doi.org/10.1371/journal.pone.0264339
Malmberg M, Ngasala B, Ferreira PE. Temporal trends of molecular markers associated with artemether-lumefantrine tolerance/resistance in Bagamoyo district, Tanzania. Malar. J. 2013; 12, 103: 10-18. https://doi.org/10.1186/1475-2875-12-103
Folarin OA, Bustamante C, Gbotosho GO, Sowunmi A, Zalis MG, Oduola AMJ, Happi CT. In vitro amodiaquine resistance and its association with mutations in pfcrt and pfmdr1 genes of Plasmodium falciparum isolates from Nigeria. Acta Trop. 2011; 120(3): 224–230.
https://doi.org/10.1016/j.actatropica.2011.08.013
Nsobya SL, Dokomajilar C, Joloba M, Dorsey G, Rosenthal PJ. Resistance-mediating Plasmodium falciparum pfcrt and pfmdr1 alleles after treatment with artesunate-amodiaquine in Uganda. Antimicrob. Agents Chemother. 2007;51(8):3023–3025. doi: 10.1128/AAC.00012-07.
Mairet-Khedim M, Leang R, Marmai C, Khim N, Kim S, Ke S, Kauy C, Kloeung N, Eam R, Chy S, Izac B, Mey Bouth D, Dorina Bustos M, Ringwald P, Ariey F, Witkowski B. Clinical and in vitro resistance of Plasmodium falciparum to artesunate-amodiaquine in Cambodia. Clin. Infect. Dis. 2021;73(3): 406–413. https://doi.org/10.1093/cid/ciaa628
Beshir K, Sutherland CJ, Merinopoulos I, Durrani N, Leslie T, Rowland M, Hallett RL. (2010). Amodiaquine resistance in Plasmodium falciparum malaria in Afghanistan is associated with the pfcrt SVMNT allele at codons 72 to 76. Antimicrob. Agents Chemother. 2010; 54(9), 3714–3716. https://doi.org/10.1128/AAC.00358-10
Echeverry DF, Holmgren G, Murillo C, Higuita JC, Björkman A, Gil JP, Osorio L Short report: polymorphisms in the pfcrt and pfmdr1 genes of Plasmodium falciparum and in vitro susceptibility to amodiaquine and desethylamodiaquine. Amer. J. Trop. Med Hyg. 2007; 77(6), 1034–1038. https://doi.org/10.4269/ajtmh.2007.77.1034
Thanh NV, Thuy-Nhien N, Tuyen NTK, Tong NT, Nha-Ca NT, Dong LT, Quang HH, Farrar J, Thwaites G, White NJ, Wolbers M, Hien TT. Rapid decline in the susceptibility of Plasmodium falciparum to dihydroartemisinin-piperaquine in the south of Vietnam. Malar. J. 2017; 16(1), 1–10.
https://doi.org/10.1186/s12936-017-1680-8
Phuc BQ, Rasmussen C, Duong TT, Dong LT, Loi MA, Ménard D, Tarning J, Bustos D, Ringwald P, Galappaththy GL, Thieu NQ. Treatment failure of dihydroartemisinin/piperaquine for Plasmodium falciparum Malaria, Vietnam. Emerg. Infect. Dis. 2017;23(4):715-717. doi: 10.3201/eid2304.161872. PMID: 28322709; PMCID: PMC5367417.
Amato R, Lim P, Miotto O, Amaratunga C, Dek D, Pearson RD, Almagro-Garcia J, Neal A T, Sreng S, Suon S, Drury E, Jyothi D, Stalker J, Kwiatkowski DP, Fairhurst RM. Genetic markers associated with dihydroartemisinin–piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype–phenotype association study. The Lancet Infect. Dis. 2017; 17(2), 164–173. https://doi.org/10.1016/S1473-3099(16)30409-1
Witkowski B, Duru V, Khim N, Ross LS, Saintpierre B, Beghain J, Chy S, Kim S, Ke S, Kloeung N, Eam R, Khean C, Ken M, Loch K, Bouillon A, Domergue A, Ma L, Bouchier C, Leang R, Ménard D. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype–genotype association study. The Lancet Infect. Dis. 2017; 17(2), 174–183. https://doi.org/10.1016/S1473-3099(16)30415-7
Win KN, Manopwisedjaroen K, Phumchuea K. Molecular markers of dihydroartemisinin-piperaquine resistance in northwestern Thailand. Malar. J. 2022; 21: 352. https://doi.org/10.1186/s12936-022-04382-5
Amaratunga C, Lim P, Suon S, Sreng S, Mao S, Sopha C, Sam B, Dek D, Try V, Amato R, Blessborn D, Song L, Tullo GS, Fay MP, Anderson JM, Tarning J, Fairhurst RM. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: A multisite prospective cohort study. The Lancet Infect. Dis. 2016;16(3): 357–365. https://doi.org/10.1016/S1473-3099(15)00487-9
van der Pluijm RW, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, Thanh NV, Jittamala P, Hanboonkunupakarn B, Chutasmit K, Saelow C, Runjarern R, Kaewmok W, Tripura R, Peto TJ, Yok S, Suon S, Sreng S, Mao S, Oun S, Dondorp AM. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. The Lancet Infect. Dis. 2019; 19(9): 952–961. https://doi.org/10.1016/S1473-3099(19)30391-3
Ahorhorlu SY, Quashie N, Ben Jensen RW, Kudzi W, Nartey ET, Odurowah N, Quashie D, Zoiku F, Dzudzor B, Wang CW, Hansson H, Alifrangis M, Adjei GO. Assessment of artemisinin tolerance in Plasmodium falciparum clinical isolates in children with uncomplicated malaria in Ghana. Malar J. 2023; 22(58): 1–10. https://doi.org/10.1186/s12936-023-04482-w
Kavishe RA, Paulo P, Kaaya RD, Kalinga A, Zwetselaar M, Van M, Chilongola J, Roper C, Alifrangis M. Surveillance of artemether-lumefantrine associated Plasmodium falciparum multidrug resistance protein-1 gene polymorphisms in Tanzania. Malar. J. 2014; 13(264): 1–6.
Plucinski MM,Talundzic E, Morton L, Dimbu PR, Macaia AP, Fortes F, Goldman I, Lucchi N, Stennies G, MacArthur JR, Udhayakumar V. Efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for treatment of uncomplicated malaria in Children in Zaire and Uíge Provinces, Angola. Antimicrob. Agents Chemother. 2015; 59:.https://doi.org/10.1128/aac.04181-14
Baraka V, Mavoko HM, Nabasumba C, Francis F, Lutumba P. Impact of treatment and re-treatment with artemether-lumefantrine and artesunate-amodiaquine on selection of Plasmodium falciparum multidrug resistance gene-1 polymorphisms in the Democratic Republic of Congo and Uganda. PLoS One. 2018; 13(2): e0191922. https://doi.org/10.1371/journal.pone.0191922
Tiwari S, Sharma N, Sharma GP, Mishra N.. Redox interactome in malaria parasite Plasmodium falciparum. Parasitol. Res. 2021; 120(2): 423–434. doi:10.1007/s00436-021-07051-9
Goyal M, Alam A, Bandyopadhyay U. Redox regulation in malaria: Current concepts and pharmacotherapeutic implications. Curr. Med. Chem. 2012; 19(10): 1475–1503. doi: 10.2174/092986712799828328.
Nepveu F, Turrini F. Targeting the redox metabolism of Plasmodium falciparum. Futur. Med. Chem. 2013;5(16):1993–2006.
Bergquist R, Elmorshedy H. Artemether and praziquantel: Origin, mode of action, impact, and suggested application for effective control of human schistosomiasis. Trop. Med. Infect. Dis. 2018;3(4): 125 doi: 10.3390/tropicalmed3040125.
Egwu CO, Augereau J, Reybier K, Benoit-vical F. Reactive oxygen species as the brainbox in malaria treatment. Antiodidants. 2021;10(12):1872. doi: 10.3390/antiox10121872.
Red I, Cells B, Siddiqui G, Giannangelo C, Paoli A, De Schuh AK, Heimsch KC, Anderson D, Brown TG, Macraild CA, Wu J, Wang X, Dong Y, Vennerstrom JL, Becker K, Creek DJ. Peroxide antimalarial drugs target redox homeostasis in Plasmodium falciparum infected red blood cells. ACS Infect. Dis. 2022;8(1):210-226. https://doi.org/10.1021/acsinfecdis.1c00550
Vasquez M, Zuniga M, Rodriguez A. Oxidative stress and pathogenesis in malaria. Front. Cell Infect. Microbiol. 2021;11: 1–8. https://doi.org/10.3389/fcimb.2021.768182
Kim JH, Chan KL, Faria NCG, Martins MDL, Campbell BC. Targeting the oxidative stress response system of fungi with redox-potent chemosensitizing agents. Front. Microbiol. 2012;3:88. doi: 10.3389/fmicb.2012.00088.
Kaiser M, Mäser P, Tadoori LP, Ioset J, Brun R. Antiprotozoal activity profiling of approved drugs: A starting point toward drug repositioning. PLoS One. 2015; 10(8):1–16. doi: 10.1371/journal.pone.0135556.
De Cremer K, De Brucker K, Staes I, Peeters A, Van Den F. Stimulation of superoxide production increases fungicidal action of miconazole against Candida albicans biofilms. Nat. Publ. Gr. 2016;5:1–14. doi: 10.1038/srep27463.
Hospital R, Road R. The antimalarial action on Plasmodium falciparum of qinghaosu and artesunate in combination with agents which modulate oxidant stress log concentration. Krung. Sud. Roch. Yong. Yuth. 1987;81:710–714.
Penna-coutinho J, Cortopassi WA, Oliveira AA, Costa TC. Antimalarial activity of potential inhibitors of Plasmodium falciparum lactate dehydrogenase enzyme selected by docking studies. PLoS One. 2011. https://doi.org/10.1371/journal.pone.0021237
Huy NT, Kamei E, Kondo Y, Serada S, Kanaori K, Takano R, Tajima K, Hara S.. Effect of antifungal azoles on the heme detoxification system of malarial parasite. J. Biochem. 2002; 131: 437–444.
Huy NT, Kamei K, Yamamoto T, Kondo Y, Kanaori K, Takano R, Tajima K, Hara S. (2002). Clotrimazole binds to heme and enhances heme-dependent hemolysis: Proposed antimalarial mechanism of clotrimazole. J. Biol Chem. 2002; 277(6): 4152–4158. https://doi.org/10.1074/jbc.M107285200
Georgewill UO, Ezerioha CE, Adikwu E. Antiplasmodial activity of ketotifen-artemether-lumefantrine on Plasmodium berghei infected mice. Int. J. Res. 2020;8(11):251–258. doi: https://doi.org/10.29121/granthaalayah.v8.i11.2020.243
Pazhayam NM, Chhibber-Goel J, Sharma A. new leads for drug repurposing against malaria. Drug Discov. Today. 2019; 24(1):263–271. doi: 10.1016/j.drudis.2018.08.006.
Grimberg BT, Mehlotra RK. Expanding the antimalarial drug arsenal-now, but how? Pharmaceuticals. 2011;4(5):681–712. doi: 10.3390/ph4050681.
Caretto S, Quarta A, Durante M, Nisi R, De Paolis A, Blando F, Mita G. Methyl jasmonate and miconazole differently affect artemisinin production and gene expression in Artemisia annua suspension cultures. Plant Biol. (Stuttg). 2011;13(1):51-58. doi:10.1111/j.1438-8677.2009.00306.x.
Vandenbosch D, Braeckmans K, Nelis HJ, Coenye T. Fungicidal activity of miconazole against Candida spp. biofilms. J. Antimicrob. Chemother. 2010;65(4):694–700. doi: 10.1093/jac/dkq019.
Nsanzabana C. Resistance to artemisinin combination therapies (ACTs): Do not forget the partner drug! Trop. Med. Inf. Dis. 2019;4(26):1-11.
Esu EB, Effa EE, Opie ON, Meremikwu MM. Artemether for severe malaria. Cochrane Database Syst. Rev. 2019: (6).
Dharavath R, Nagaraju N, Reddy MR, Ashok D, Sarasija M, Vijjulatha M, Prashanthi G. Microwave-assisted synthesis, biological evaluation and molecular docking studies of new coumarin-based 1, 2, 3-triazoles. RSC Adv. 2020;10(20):11615-11623.
Akpa PA, Ugwuoke JA, Attama AA, Ugwu CN, Ezeibe EN, Momoh MA, Echezona AC, Kenechukwu FC. Improved antimalarial activity of caprol-based nanostructured lipid carriers encapsulating artemether-lumefantrine for oral administration. Afr. Health Sci. 2020; 20(4):1679-1697.
https://dx.doi.org/10.4314/ahs.v20i4.20
Adeyemi OI, Ige OO, Akanmu MA, Ukponmwan OE. In vivo anti-malarial activity of propranolol against Plasmodium berghei ANKA infection in mice. Afr. J. Exper. Microbiol. 2020;21(4):333-339.
Waknine-Grinberg JH, Even-Chen S, Avichzer J, Turjeman K, Bentura-Marciano A, Haynes RK, Weiss L, Allon N, Ovadia H, Golenser J, Barenholz Y. Glucocorticosteroids in nano-sterically stabilized liposomes are efficacious for elimination of the acute symptoms of experimental cerebral malaria. PLoS One. 2013; 8, e72722. https://doi.org/10.1371/journal.pone.0072722.
Borhade V, Pathak S, Sharma S, Patravale V. Clotrimazole nanoemulsion for malaria chemotherapy. Part II: Stability assessment, in vivo pharmacodynamic evaluations and toxicological studies. Int. J. Pharm. 2012;431(1–2):149–160. doi: 10.1016/j.ijpharm.2011.12.031.
Georgewill UO, Adikwu E. Potential antimalarial activity of artemether-lumefantrine-doxycycline: A study in mice infected with Plasmodium berghei. Adv. Pharm. J. 2021;6(1):22-28.
Shafi S, Gupta S, Jain R, Shoaib R, Munjal A, Maurya P, Najmi AK, Singh S. Tackling emerging artemisinin resistance by modulating the defensive oxido-reductive mechanism of human malaria parasite by repurposing nitrofurantoin. BioRxiv preprint: 1-26. doi: https://doi.org/10.1101/2023.04.18.537303
Lefèvre G, Thomsen MS. Clinical pharmacokinetics of artemether and lumefantrine (Riamet®). Clin. Drug. Investig. 1999;18:467–480.
Umeyor CE, Okoye I, Uronnachi EM, Okeke T, Kenechukwu FC, Attama AA. Repositioning miconazole nitrate for malaria: Formulation of sustained release nanostructured lipid carriers, structure characterization and in vivo antimalarial evaluation. J. Drug Deliv. Sci. Tech. 2021;61(102125):1-11. https://doi.org/10.1016/j.jddst.2020.102125
Tripathi R, Rizvi A, Pandey SK, Dwivedi H, Saxena JK. Ketoconazole, a cytochrome P450 inhibitor can potentiate the antimalarial action of α/β arteether against MDR Plasmodium yoelii nigeriensis. Acta Trop. 2013;126:150–155.
Bravo Gonza´lez RC, Huwyler J, Boess F, Walter I, Bittner B. In vitro investigation on the impact of the surface-active excipients - cremophor EL, Tween® 80 and Solutol® HS 15 on the metabolism of midazolam. Biopharm. Drug Disp. 2004;25:37–49.
Christiansen A, Backensfeld T, Denner K, Weitschies W. Effects of non-ionic surfactants on cytochrome P450-mediated metabolism in vitro. Eur. J.
Pharm. Biopharm. 2011;78:166–72.
Nnamani PO, Kenechukwu FC, Nwagwu SC, Okoye O, Attama AA. Physicochemical characterization of artemether-entrapped solid lipid microparticles prepared from templated-compritol and Capra hircus (goat fat) homolipid. Dhaka Univ. J. Pharm. Sci. 2021;20(1):67-80. DOI: https://doi.org/10.3329/dujps.v20i1.54034
Momoh MA, Kenechukwu FC, Ugwu CE, Adedokun MO, Agboke AA, Agbo CP, Ossai EC, Ofomata AC, Youngson DC, Omeje CE, Amadi BC. Development and evaluation of artemether-loaded microspheres delivery system for oral application in malaria treatment. Trop. J. Nat. Prod. Res. 2021; 5(11):2030-2036. http://doi.org/10.26538/tjnpr/v5i11.23
Nnamani PO, Kenechukwu FC, Omeje MA, Nwachukwu LO, Anazodo FI, Nwagwu CS, Kola-Mustapha, AT, Obitte NC, Attama AA. Development and characterization of sustained-release artemether-loaded solid lipid microparticles based on mixed lipid core and a polar heterolipid. Afri J Pharm Res Dev. 2022;14(1):1-17.
Attama AA, Kenechukwu FC, Onuigbo EB, Nnamani PO, Obitte NC, Finke JH, Pretor S, Muller-Goymann CC. Solid lipid nanoparticles encapsulating a fluorescent marker (coumarin 6) and anti-malarials – artemether and lumefantrine: evaluation of cellular uptake and anti-malarial activity. Eur. J. Nanomed. 2016;8:129–138.
Kenechukwu FC, Neto RPC, Dias ML, Ricci-Junior E. Compatibilized biopolymer-based core-shell nanoparticles: A new frontier in malaria combo-therapy. J. Pharm. Innov. 2022; Early online:1-27. DOI: https://doi.org/10.1007/s12247-022-09664-8
Nardos A, Makonnen E. In vivo antiplasmodial activity and toxicological assessment of hydroethanolic crude extract of Ajuga remota. Malar. J. 2017;16:25:1-8.
Nnamani PO, Ugwu AA, Ibezim EC, Kenechukwu FC, Akpa PA, Ogbonna JDN, Obitte NC, Odo AN, Windbergs M, Lehr CM, Attama AA. Sustained-release liquisolid compact tablets containing artemether-lumefantrine as alternate-day regimen for malaria treatment to improve patient compliance. Int. J. Nanomed. 2016;11:6365-6378.
Agbo CP, Umeyor CE, Kenechukwu FC, Ogbonna JDN, Chime SA, Charles L, Agubata CO, Ofokansi KC, Attama AA. Formulation design, in vitro characterizations and anti-malarial investigations of artemether and lumefantrine-entrapped solid lipid microparticles. Drug Dev. Ind. Pharm. 2016;42(10):1708-1721.
Ogbonna JDN, Echezona AC, Nwagwu CS, Agbo CP, Onugwu AL, Kenechukwu FC, Akpa PA, Momoh MA, Attama AA. Formulation, in vitro and in vivo evaluation of sustained released artemether-lumefantrine-loaded microstructured solid lipid microparticles (SLMs). Trop. J. Nat. Prod. Res. 2021;5(8):1460-1469. http://doi.org/10.26538/tjnpr/v5i8.23
Ogbonna JDN, Nzekwe IT, Kenechukwu FC, Nwobi CS, Amah JI, Attama AA. Development and evaluation of chloroquine phosphate microparticles using solid lipid as a delivery carrier. J. Drug Discov. Dev. Deliv. 2015; 2(1): 1011.