Momordica charantia Leaf Extract Attenuates Cyanide Toxicity by Enhancing Antioxidant, Anti-inflammatory and Cholinergic response in Rats’ Brain Tropical Journal of Natural Product Research
Main Article Content
Abstract
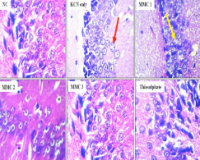
Medicinal plants have been exploited over centuries for the management and treatment of diseases and as antidote against acute poisoning. Here, the antioxidant, anticholinergic and anti-inflammatory impacts of methanol extract of Mormodica charantia (MMC) leaf were investigated in the cortex and hippocampus of cyanide-intoxicated rats. Except for animals in control and potassium cyanide (KCN) groups that were orally administered distilled water and KCN, other groups were co-administered 0.4 mg/kg of freshly prepared KCN and varying dose (100, 200 and 400 mg/kg) of MMC and 200 mg/kg thiosulphate. Animals were treated for 21 days and thereafter sacrificed for sample collections for biochemical and histopathological studies. Findings from this study revealed enhanced reversal of oxidative stress markers near normalcy in rats’ brain cortex and hippocampus. Acetylcholine esterase (AChE) and butyrylcholine esterase (BChE) activities were significantly reduced (p<0.05) in animals that were administered varying doses of MMC and thiosulphate relative to KCN untreated group. The level of hippocampal TNF–α and IL–6 significantly decreased (p<0.05) in MMC treated animals compared to KCN groups. Micromorphology section of both brain and hippocampus revealed improvement in MMC treatment as no degenerative changes was observed in these sections. This study has demonstrated that Mormodica charantia methanol extract is medicinally potent against cyanide toxicity and presumably contain lead compound that could be harnessed for the treatment of oxidative damage, inflammatory and neuronal responses in cyanide poisoning.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Zuhra K, Szabo C. The two faces of cyanide: an environmental toxin and a potential novel mammalian gasotransmitter. Fed Euro Biochem Soc J 2022; 289: 2481–2515. https://doi.org/10.1111/febs.16135
Hariharakrishnan J, Satpute RM, Bhattacharya R. Cyanide-induced changes in the levels of neurotransmitters in discrete brain regions of rats and their response to oral treatment with a-ketoglutarate. Indian J exp bio 2010; 48: 731-736.
Husain K, Ansari RA, Ferder L. Pharmacological agents in the prophylaxis/treatment of organo-phosphorous pesticide intoxication. Indian J Exp Biol 2010; 48(7): 642-650.
Gitonga F, Biwott K, Gitau GW, Wafula OP, Amwayi P, Isaac AO, Nyariki JN. Coenzyme Q10 Ameliorates potassium cyanide-induced toxicosis in a mouse model. Sci African, 2021; 12, e00815. https://doi.org/10.1016/j.sciaf.2021.e00815
Adamolekun B. Neurological disorders associated with cassava diet: a review of putative etiological mechanisms. Metab Brain Dis. 2011; 26: 79–85. https://doi.org/10.1007/s11011-011-9237-y
Zhang D, Lee B, Nutter A, Song P, Dolatabadi N, Parker J, Sanz-Blasco S, Newmeyer T, Ambasudhan R, McKercher SR., Masliah E, Lipton SA. Protection from cyanide‐induced brain injury by the Nrf2 transcriptional activator carnosic acid. J Neurochem 2015; 133(6): 898-908. https://doi.org/10.1111/jnc.13074
Alqahtani RM, Alyousef MY, Alwatban ZH, Ghandour MK. Long-Term Neuropsychiatric Sequelae in a Survivor of Cyanide Toxicity Patient with Arterialization. Cureus 2020; 12(6): e8430. https://doi.org/10.7759%2Fcureus.8430
Borron WS, Baud JF. Antidotes for acute cyanide poisoning. Curr Pharm Biotechnol 2012; 13(10): 1940–1948 https://doi.org/10.2174/138920112802273182
Muhammad, A., Muhammad, S.K., Muhammad, A.K., Kamran, A., Zahoorul, H., Jawaria AK, Nisar A. Assessing the efficacy of aqueous garlic extract, sodium nitrite and sodium thiosulfate against prolonged oral cyanide exposure in Rabbits, Pak J Pharm Sci 2018; 31(2): 411–419
Kadiri HE, Okoro IO, Ichipi-Ifukor PC. Tetrapleura Tetraptera Fruit Protects Against Cyanide Induced Toxicity in Rats. Iraqi J Sci 2020; 61(10): 2504–2514. https://doi.org/10.24996/ijs.2020.61.10.7
Bolaji OM, Olabode OO. Modulating effect of aqueous extract of Telfairia occidentalis on induced cyanide toxicity in rats. Niger J Physiol Sci. 2011; 26: 185–191
De Campos OC, Layole MP, Iheagwam FN, Rotimi SO, Chinedu SN. Effects of aqueous extract of Momordica charantia on survival, locomotive behavior, and antioxidant status of Drosophila melanogaster. Trop J Nat Prod Res. 2021; 5(1): 178-181. doi.org/10.26538/tjnpr/v5i1.23
Fan M, Kim E-K, Choi Y-J, Tang Y, Moon S-H. The Role of Momordica charantia in resisting obesity. Inter J Environ Res Public Health 2019; 16(18): 3251–3251. https://doi.org/10.3390/ijerph16183251
Ishola I, Akinyede A, Sholarin A. Antidepressant and Anxiolytic Properties of the Methanolic Extract of Momordica charantia Linn (Cucurbitaceae) and its Mechanism of Action. Drug Res 2013; 64(07): 368–376. https://doi.org/10.1055/s-0033-1358712
National Research Council. Occupational Health and Safety in the Care and Use of Research Animals. Washington, DC: The National Academies Press 1997. https://doi.org/10.17226/4988.
Ofuegbe SO, Oyagbemi AA, Omobowale TO, Adedapo AD, Ayodele AE, Yakubu MA, Oguntibeju OO, Adedapo AA. Methanol leaf extract of Momordica charantia protects alloxan-induced hepatopathy through modulation of caspase-9 and interleukin-1β signalling pathways in rats. 2020; www.veterinaryworld.org/Vol.13/August-2020/6.pdf
Bradford KM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt Biochem 1976; 72(1-2): 248-254. https://doi.org/10.1016/0003-2697(76)90527-3
Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Ann Biochem 1970; 34(1): 30–38
Nyman M. Serum hatoglobin; methodological and clinical studies. Scandinavian J Clini Lab Investi. 1959; 11(Supp 39): 1–169.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82: 70–77.
Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972; 247(10): 3170–3175
Buege JA, Aust SD. Microsomal lipid peroxidation. Meth Enzymol 1978; 52: 302–310. https://doi.org/10.1016/S0076-6879(78)52032-6
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmaco 1961; 7(2): 88–95.
Fasco MJ, Hauer CR, Stack RF, O’Hehir C, Barr JR, Eadon GA. Cyanide Adducts with Human Plasma Proteins: Albumin as a Potential Exposure Surrogate. Chem. Res. Toxicol. 2007; 20: 677–684. https://doi.org/10.1021/tx6003425
Sabourin PJ, Kobs CL, Gibbs ST, Hong P, Matthews CM, Patton KM, Sabourin CL, Wakayama EJ, Characterization of a mouse model of oral potassium cyanide intoxication. Int. J. Toxicol. 2016; 35(5): 584–603.
Zhang F, Lin L, Xie J. A mini-review of chemical and biological properties of polysaccharides from Momordica charantia. Inter J Bio Macromol 2016; 92: 246–253. https://doi.org/10.1016/j.ijbiomac.2016.06.101
Maneenin C, Burawat J, Maneenin N, Nualkaew S, Arun S, Sampannang A, Iamsaard S. Antioxidant capacity of Momordica charantia extract and its protective effect on testicular damage in Valproic acid-induced rats. Int J Morphol 2018; 36(2): 447–453. http://dx.doi.org/10.4067/S0717-95022018000200447
Wang L, Clardy A, Hui DF, Gao AW, Wu Y. Antioxidant and antidiabetic properties of Chinese and Indian bitter melons (Momordica charantia L.). Food Biosci 2019; 29: 73–80. https://doi.org/10.1016/j.fbio.2019.03.010
Akintimehin ES, Karigidi KO, Omogunwa TS, Adetuyi FO. Safety assessment of oral administration of ethanol extract of Justicia carnea leaf in healthy wistar rats: hematology, antioxidative and histology studies. Clin. Phytosci. 2021; 7: 2. https://doi.org/10.1186/s40816- 020- 00234-4
Tshala-Katumbay DD, Ngombe NN, Okitundu D, David L, Westaway SK, Boivin MJ, Mumba ND, Banea JP. Cyanide and the human brain: perspectives from a model of food (cassava) poisoning. Ann N Y Acad Sci. 2016; 1378(1): 50–57. https://doi.org/10.1111/nyas.13159
Blake MG, Krawczyk MC, Baratti CM, Boccia MM. “Neuropharmacology of memory consolidation and reconsolidation: insights on central cholinergic mechanisms.” J Physiol Paris. 2014; 108(4–6): 286–291. https://doi.org/10.1016/j.jphysparis.2014.04.005
Park HR, Lee H, Park H, Cho WK, Ma JY. “Fermented Sipjeondaebo-tang alleviates memory deficits and loss of hippocampal neurogenesis in Scopolamine-induced amnesia in mice. Sci Rep. 2016; 6: 22405. https://doi.org/10.1038/srep22405
Chen F, Jiang L, Yang B. Visual loss caused by acute cyanide poisoning: a case report. Clin Toxicol 2011; 49(2): 121–123. https://doi.org/10.3109/15563650.2011.556643
Martins NO, de Brito IM, Araujo SSO, Negri G, Carlini EA, Mendes FR. Antioxidant, anticholinesterase and antifatigue effects of Trichilia catigua (catuaba). BMC Complement Alternat Med 2018; 18: 172. https://doi.org/10.1186/s12906-018-2222-9
Kayode AAA, Kayode OT, Rotimi DE. Pharmacological actions of phytoconsitutnts on neurodegenration disorders. Trop J Nat Prod Res. 2022; 6(7): 1019-1046. https://10.26538/tjnpr/v6i7.2
Valarmathi N, Sree RS, Rajan TS. Neuroprotective effects of Momordica charantia: A review from preclinical Studies. Int J Res Pharm Sci 2020; 11(2): 1902–1907
Zhu L, Xu YJ, Du F, Qian ZM. Ginkgolides protect primary cortical neurons from potassium cyanide-induced hypoxic injury. Exp Brain Res 2007; 179(4): 665–671. https://doi.org/10.1007/s00221-006-0823-x
Ozel AB, Cilingir-Kaya OT, Sener G, Ozbeyli D, Sen A, Sacan O, Yanardag R, Yarat A. Investigation of possible neuroprotective effects of some plant extracts on brain in bile duct ligated rats. J Food Biochem 2021; 45: e13835. https://doi.org/10.1111/jfbc.13835
Collins T, Cybulsky MI. Firdausi SR, Nur’aini RAR, Izzah FN, Nabilah SN, Christiana YI, Dwijayanti DR, Rahayu S, Rifa IM, Djati MS. Elephantopus scaber ethanol extract suppresses inflammation via regulation of the NF-Kb pathway expression in pulmonary fibrosis. Trop J Nat Prod Res. 2024; 8(9): 8554-8560 https://doi.org/10.26538/tjnpr/v8i9.44
Zhu L, Chen T, Chang X, Zhou R, Luo F, Liu J, Zhang K, Wang Y, Yang Y, Long H, Liu Y, Yan T, Ma C. Salidroside ameliorates arthritis-induced brain cognition deficits by regulating rho/ROCK/ NF-kappaB pathway. Neuropharmaco 2016; 103: 134–142. https://doi.org/10.1016/j.neuropharm.2015.12.007
Gong J, Sun F, Li Y, Zhou X, Duan Z, Duan F, Shen J. Momordica charantia polysaccharides could protect against cerebral ischemia/reperfusion injury through inhibiting oxidative stress mediated c-Jun N-terminal kinase 3 signaling pathway. Neuropharmacol 2015; 91: 123–134.
https://doi.org/10.1016/j.neuropharm.2014.11.020
Qaid HR,Aljunaid MA, Kaid N, Ridwan RD, Budi HS, Diyatri I, Kamila NT, Alkadasi BA, Alaghbari SG, AAlqhtani AS. Epigallocatechin-3-gallate modulates NF-kB in Porphyromonas gingivalis-induced periondotitis. Trop J Nat Prod Res. 2024; 8(8): 8032-8036. https://doi.org/10.26538/tjnpr/v8i8.15
Nerurkar PV, Johns LM, Buesa LM, Kipyakwai G, Volper E, Sato R, Shah P, Feher D, Williams PG, Nerurkar VR. Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuro-inflammation. J Neuroinflamm 2011; 8(1): 64. https://doi.org/10.1186/1742-2094-8-64
Deng Z, Yuan C, Yang J, Peng Y, Wang W, Wang Y, Gao W. Behavioural defects induced by chronic social defeat stress are protected by Momordica charantia polysaccharides via attenuation of JNK3/PI3K/AKT neuroinflammatory pathway. Ann Transl Med 2019; 7(1): 6. https://doi.org/10.21037%2Fatm.2018.12.08