Inhibition of Dehydrogenase Activity in Sputum Bacterial Isolates by hot aqueous extracts of selected Nigeria anti-cough plants Tropical Journal of Natural Product Research
Main Article Content
Abstract
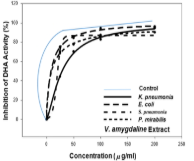
The exploration of plant-based medications as primary therapeutic agents for the treatment of various ailments is currently ongoing. The present study evaluates the antimicrobial properties of hot aqueous leaf extracts of Piper guineense, Gongronema latifolium, and Vernonia amygdalina against clinical sputum isolates. The isolates were characterised based on morphology and biochemical properties. The dehydrogenase enzyme assay and the Agar-well diffusion techniques were used in evaluating the antimicrobial activity of plants, singly and in combination at ratios of 1:1:1. Streptococcus species (46.16%), Klebsiella species (23.08%), Escherichia coli (15.38%), and Proteus species (15.38%) were all present in the bacteria isolates at high prevalence rates. The aqueous extract of V. amygdalina produced the highest level of antimicrobial activity, followed by the combined extract, which inhibited the growth of Klebsiella spp and Escherichia coli at 200 mg/mL. At 200 mg/mL, G. latifolium and P. guineense inhibited Streptococcus species with a 14 mm-diameter zone. When compared to the standard drug, Gentamicin, results of the dehydrogenase activity assay indicate that V. amygdalina and the combined extract exhibited the most favourable IC50 values of 8.69 g/mL, and 9.04 g/mL, 6.37 g/mL, and 9.87 g/mL against E. coli and Proteus spp, respectively. The high IC50 values indicated that Klebsiella spp resisted the plant extracts considerably. Plant aqueous extracts have shown that they are potential natural drug agents for treating and managing cough-causing organisms.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Shakya A.K. Medicinal plants: future source of new drugs. Int J. of Herbal Med. 2016;4(4) 59-64
Newman DJ, Cragg GM, Kingston DG. In the practice of medicinal chemistry,4th Ed.C.G. Wermuth et al. (Eds.), Elsevier: Amsterdam 2015. 101–139 p.
Sarker SD, Nahar L. Chemistry for pharmacy students’ general, organic and natural Product Chemistry, John Wiley and Sons, 2007. 283-359 p.
Siemieniuk RA, Gregson D.B, Gill M.J. The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study, BMC Infect. Dis. 2011;11-314
Alisi CS, Nwanyanwu CE, Akujobi CO, Ibegbulem CO. Inhibition of Dehydrogenase activity in pathogenic bacteria isolates by aqueous extracts of Musa paradisiaca (Var Sapientum) Afri. J. of Biotech. 2008;7 (12): 1821-1825
Quintas V, Prada-López I, Tomás I. Analysing the oral biofilm using fluorescence-based Microscopy: what’s in a dye? In: A. Mendez Vilas (Ed.), Microscopy: Adv. in Sci. Res and Edu.2014;226-238
Xie J. Detection of amount and activity of living algae in freshwater by dehydrogenase Activity DHA. Environ Mon and Ass. 2008; 146: 473-478.
Offor CE, Nweke FN., Okaka AN, Igwenyi, IO. Onwe V.N. Analysis of the antinutrients levels in staple food crops in three different local government areas of Ebonyi state, Nigeria. Conti. J. Food Sci and Techn. 2011; 5 (1): 26 – 30
Todar, K. The Normal Bacterial Flora of Humans. TODAR’S Online Textbook of Bacteriology. 2008;1-5 p.
Igwe CU, Onyeagoro EE, Morah AC, Nwaogu LA, Iheme CI, Onwuliri VA. Phenolic profile and in vitro antioxidant potential of four medicinal plants. Trop. J. Nat. Prod. Res 2021;5(11): 2037-2042.
Moundipa FP, Kamini G, Melanie F, Bilong FC, Bruchhaus I. In vitro amoebic Activity of some medicinal plants of the Bamun region (Cameroon) Afri. J. of Trad Cam. 2000; 62:113–121
Hladik C, Krief S, Haxaire C. Ethnomedicinal and bioactive properties of plants ingested by wild chimpanzees in Uganda. J. Ethnopharmacol. 2005; 101-115.
Muraina IA, Adaudi AO, Mamman M, Kazeem HM, Picard J, McGaw LJ, Eloff JN Antimycoplasmal activity of some plant species from northern Nigeria compared to the currently used therapeutic agent. Pharma. Bio. 2010; 48:1103–1107
Masaba S.C. Antimicrobial activity of Vernonia amygdalina Del. Trans R Soc Trop Med Hyg. 2000; 694 -695
Nwozo SO, Orojobi BF, Adaramoye OA. Hypolipidemic and antioxidant potentials of Xylopia aethiopica seed extract in hypercholesterolemic rats. J Med Food. 2011 ;14(1-2):114-119. doi: 10.1089/jmf.2008.0168. PMID: 21244241.
Osuagwu OL, Igwe CU, Nzebude CP, Njoku OC, Ejiofor JC. Phenolic profile and antioxidant potential of aqueous extracts of selected traditional anti-cough plants. J. Drug Deliv. Ther. 2023; 13(7); 22-29.
Ogunniran KO. Antibacterial effects of extracts of Ocimum gratissimum and piper guineense on Escherichia coli and Staphylococcus aureus. Afri. J. of Food Sci. 2009; 3:77–81.
Juliani HR, Simon JE, Ho CT. Piper guineense (Piperaceae): Chemistry, Traditional Uses and Functional Properties of an “African Black Pepper” In book: African Natural Plant Products Volume II: New Discoveries in Chemistry, Health and Nutrition Edition: Symposium Series 1127Publisher: American Chemical Society (ACS). 2013; 1127:33-48.
Morebise Olugbenga. A Review on Gongronema latifolium, an Extremely Useful Plant with Great Prospects. Euro. J. of Med. Plants. 2015; 10(1): 1-9
Afolabi FE. Chemical composition and antibacterial activity of Gongrenema latifolium. J. Zhejiang Univ. Biol. Sci. 2007; 8(5):356-357.
Essien JP, Ebong GA, Akpan EJ. Antioxidant and antitussive properties of Gongronema latifolium leaves used locally for the treatment of fowl cough in Nigeria. J.Appli. Sci. in Enviro. Mgt. 2007; 11(4):47-50
Mosango D.M. Gongronema latifolium Benth. Record from PROTA4u.Schmelzer GH, Gurib-Fakim A (eds). Plant Resources of Tropical Africa (PROTA);2011. Available https://www.prota4u.org/search.asp.
Fawole, M.O, Oso, B.A. Characterisation of Bacteria: Laboratory Manual of Microbiology. 4th Edn., Spectrum Book Ltd., Ibadan, Nigeria, 2004; 24-33 p.
Oberoi A, Aggarwal A. Bacteriological Profile, Serology and Antibiotic Sensitivity Pattern of Micro-organisms from Community-Acquired Pneumonia. JK Science. 2006; 8(2):79–82
Bijendra Raj Raghubanshi, Bal Man Singh Karki. Bacteriology of Sputum Samples: A Descriptive Cross-sectional Study in a Tertiary Care Hospital. J. Nepal Med. Ass. 2020; 58(221): 24–28
Biswas B, Rogers K, McLaughlin F, Daniels D, Yadav A. Antimicrobial activities of leaf extracts of guava (Psidium guajava L.) on two gram-negative and gram-positive bacteria. Int. J. Microbiol. 2013;2013(1):746165.
Akujobi CO, Ogbulie JN, Uchegbu UN. Antibacterial activities and preliminary phytochemical Screening of Vernonia amygdalina and Citrus aurantifolia. Nig. J. of Microbes. 2006; 20(1): 649-654.
Njoku HO, Akujobi CO, Ogbulie J.N. Inhibition of dehydrogenase activity in bacterial isolates from palm wine by extracts of Vernonia amygdalina. Int. J. Chem. Biol. 2010;4(4):1093-1110. https://doi.org/10.4314/ijbcs.v4i4.63046
Osadebe PO, Ukwueze SE. A comparative study of the phytochemical and antimicrobial properties of the Eastern Nigerian species of African mistletoe (Loranthus micronthus sourced from different host trees. J. of Bio Res and Biotech. 2004; 2(1): 18-23
Nweke CO, Okolo JC, Nwanyanwu CE, Alisi CS. Response of planktonic bacteria of New Calabar River to zinc stress. Afri. J. of Biotech. 2006; 5(8): 653-658.
Ntite UF. Determination of antimicrobial potentials of ethanol extract of Combretum dolichopentalum leaves by total dehydrogenase activity assay. Int. J. Pharm. Phytochem. Ethnomed. 2017;27(8): 27-40